from Leadership Medica n. 278/2009
New therapeutic option
The increasing appearance of a reduced blood supply to the limbs is due to the presence of chronic arteriopathy of lower limbs and it represents an expression of severe atherosclerosis with high risk of amputation. In fact, chronic arteriopathy of lower limbs can eventually lead, in some patients, to amputation after a series of stages, which are: onset of rest pain, non-healing skin injuries, ulcerations, gangrene and super-infections of ischaemic lesions. Critical ischaemia itself is characterized by the presence of rest pain, difficulty in walking and loss of substance in the lower limbs, leading to amputations. Appearance of symptoms of claudication (limping) and prevention of the progression of atherosclerotic disease towards even worse degrees of it, is based on the improvement of circulation, before trophic lesions of the lower limbs appear.
This is why, first of all, it is important to modify the life style by quitting cigarette smoking, increasing physical exercise and losing weight; secondly, it is important to treat high blood pressure, cholesterol, diabetes mellitus and chronic renal failure. In more severe cases of arteriopathy we are obliged to save the limb through procedures that improve, directly and quickly, peripheral vascularisation. Surgery and intravascular techniques can be performed. Surgical techniques aim at removing obstructions or supplying blood by creating a by-pass. Intravascular re-vascularisation techniques have been recently developed and allow, through a series of new catheters, the re-opening of closed blood vessels: these techniques are cryo-angioplasty, angioplasty, stenting, atherotomy with rotablator. This sudden increase of medical and surgical devices has determined, between 1998 and-2003, a reduction of amputation rate by 21%. The development of these techniques and alternative methods of re-vascularisation are urgently needed to reduce the number of amputations and, therefore, the devastating impact on life expectancy and life quality.
Physiopathology of critical ischaemia
Patients with critical ischemia show a blood flow reduction in the lower limbs. This can be assessed through the measurement of the ratio between blood pressure measured in the lower and in the upper limbs. Normally, blood pressure in the lower limbs is over 100 mmHg. When the perfusion pressure in the lower limbs is reduced, also the oxygen supply to the tissues is reduced. Thus, muscles and skin that don't get enough blood and oxygen supply, start showing clinical signs of ischaemia. One of the first clinical signs of ischaemia is claudication (limping), that depends on insufficient blood and oxygen supply to the muscles during walking and physical exercise. Pain from claudication also hampers the ability of walking and it disappears after a few minutes of rest. If the pattern of ischaemia is more severe, the pain appears not only under stress, but also at rest and it is localized in the lower limbs. If the blood pressure in the lower limbs remains under 60 mmHg for a prolonged period of time, we then have a picture of hypoperfusion which will lead to necrosis and ulceration. The onset, then, of a clinical picture of critical ischaemia requires a quick diagnostic and therapeutic measure in order to save the affected limb. Only 50 % of patients with severe and advanced critical ischaemia can be re-vascularised; 25 % are treated with medical therapy and dressings, whereas the remaining 25 % are eligible for amputation. These patients show a number of other diseases: ischaemic heart disease, chronic obstructive broncho-pneumopathy (COBP), diabetes, chronic renal failure which account for high mortality (20 - 40 %) within 12 months from the onset of the acute episode in the lower limbs (1). Despite several modern therapeutic options, 25 % of patients will die within the first year, 30 % will show improvement, 25 % will undergo amputation and 20 % will experience worsening of critical ischaemia. In fact, of all patients treated with major amputations, only 32 % after one year from the operation will be able to walk with a prosthesis (3). Surely, diabetic patients and smokers have a much higher probability of undergoing amputation once they start showing symptoms of critical ischaemia compared to patients with high blood pressure and high lipid levels in the blood(4).
New therapeutic options in the treatment of critical ischaemia
The aim of the treatment of critical ischaemia of lower limbs is to obtain the improvement of blood flow to the impaired limb. More than 50% of patients is surgically treated with classic surgery or intravascular procedures. (5) Patients affected by this kind of pathology ,but who are not eligible for surgical or intravascular procedures, have been considered, up to now, responsive to medical treatment such as vasodilators- namely prostanoids, which have been poorly successful since they determined an improvement of the clinical picture only in 50% of all cases (6).
Therefore, we searched for new therapeutic options to relieve pain, to improve walking and reduce the number of amputations in patients with critical ischaemia who cannot be re-vascularised. We studied genetic and cellular mechanisms of stimulation or suppression of the growth of new vessels. All this seems to be really promising and this is why we are trying to start a treatment based on stem cells. An example is the research made by J. Isner et al, who have seen that the growth of new vessels is a common phenomenon in adults and it is the factor that mainly rules a series of events like tumour growth, healing of wounds and the response to ischaemia in muscle cells of lower limbs and heart. (7). This research has related the concept of angiogenesis to the possibility that haemopoietic cells can differentiate into endothelial vascular cells in areas of vascular remodelling. Isner's idea is that the injection of endothelial progenitor cells (EPC) taken from the bone marrow and injected in to the areas of ischaemia, guided by cytokine signals, can turn into endothelial vascular cells (EC) as a part of formation of new vessels. This process is called new-vascularization (8,9).
Recent therapeutic options in the treatment of critical ischaemia of lower limbs: cell therapy
Both in the bone marrow and in the circulating blood mononucleated cells (MNC) have been found, among which endothelial progenitor cells (EPCs). Recent evidence shows that these cells can differentiate both into endothelial and muscular cells (8,9,10,11). This characteristics, together with a double localization (bone marrow and peripheral blood) has lead to a new concept of formation of new blood vessels , starting from the classic concept of angiogenesis, which means the formation of new blood vessels from pre-existing endothelial cells through processes of cell migration and differentiation (11.,We get to the concept of vasculogenesis , which is referred to the in situ formation of new blood vessel starting from endothelial progenitor cells (EPC), which migrate from the bone marrow to the ischaemic areas and lead to the formation of adult cells. All this occurs through processes of adhesion, proliferation, differentiation and release of mediators (8,12,13). Both bone marrow and circulating blood EPCs, show surface markers such as CD 133 and CD34 (10,12). They are indistinguishable from haemopoietic stem cells until they are stimulated by cellular signals leading to differentiation of the muscular or endothelial line. In fact, CD34+ are multi-powered cells and can differentiate along both maturating lines(9). The bone marrow, moreover, other than cells that can determine cell and tissue regeneration, also has accessory cells which sustain angiogenesis and vasculogenesis, by producing cytokines and growth factors. These molecules are essential in the process of cellular differentiation of EPCs.
EPCs are present in the peripheral blood of patients with endothelial damage, i.e. patients with atherosclerosis, apparently in order to repair the damage. It has been shown that the action of nucleated cells of peripheral blood is less powerful than that of the same cells taken from the bone marrow(14). The possible therapeutic effect of MPC taken from the bone marrow is due not only to their number and type, but pre-clinical studies have assessed their importance in promoting the release of several cytokines that induce the development of collateral arteries in ischaemic animal models, both in the circulating blood and in the myocardium (15). In particular, it has been shown that VEGF promotes mobilization of endothelial progenitors induced by ischaemia or by other growth factors and cytokines and coordinates their proliferation and differentiation of these cells into endothelial and adult muscular cells (8,13,14).
Hence, cells extracted from bone marrow, among which there are EPCs, help the process of new-vascularisation directly through the formation of smooth muscle cells and adult endothelial cells, and indirectly through the release of several cytokines and growth factors that stimulate this process(9,10,11,16). Therefore, the presence of cell progenitors and cytokines seems to have therapeutic effects by restoring regular blood supply to ischaemic tissues. Several in vivo- studies on animal models have fully shown the positive effect of MPCs taken from bone marrow in the treatment of ischaemic tissues through the formation of collateral vessels.(17,18). Both models of myocardial and peripheral ischaemia have been studied (19,20,21) and they all demonstrated safety and efficacy of treatment by the use of these techniques. Also several studies of patients and one randomized study have shown the therapeutic possibilities and the safety of transplant of cells from autologous bone marrow in patients with critical lower limbs ischaemia (22,23,24); especially the study conducted by Tateishi et al, with 47 patients with critical lower limbs ischaemia . Those patients could not undergo surgical revascularisation but were treated with concentrated cells from autologous bone marrow which showed, versus cases treated with placebo, a considerable improvement of blood flow. This was proved by a great improvement in walking, enhanced oxygen tissue saturation and prominent reduction of pain. Regards the techniques of extraction of these cells from bone marrow, several evidences show that the defined "Harvest BMAC system" allows to obtain better results if compared to classical methods (Ficoll abd Harvest diluted). Such technique separates and concentrates the whole set of nucleated cells present in the marrow aspirate. It is a quick and non-operator dependent technique and it keeps in the cellular sediment platelets and granulocytes that have a positive effect especially in the production and release of cytokines and pro-angiogenetic growth factors ( mainly VEGF which stimulates CD34+ cells to produce adult endothelial cells starting from EPCs). Since it has been demonstrated by Heeschen et al that the migrating capability of BM-MNCs remains the only prediction factor, independent for a new-angiogenesis in models of animal critical ischaemia (24,25) and that this actual capability is preserved by a minimal manipulation that the cells undergo during and after being taken from the bone marrow, Harvest's system guarantees a minimal manipulation and minimal functional alteration of those cells and it is ,therefore, to be preferred to other methods.
As far as the optimal cell concentration, the only randomized controlled study implies the administration of a quantity between 0.7 and 2.8x10Ù7 of cells and 3.4x10Ù9 CD34+ of stem cells. These cells are processed, starting from a minimum of 500 ml of marrow aspirate(26). This study proposes an optimal concentration of 3.56x10Ù9 of nucleated cells with a range between 2.37 and 4.76x10Ù9. This number is higher than the top limit of the range used in the Tateiscìshi-Yuyama's study and it can be reached by taking 240 ml of marrow aspirate.
Treatment
The aim of this treatment is to demonstrate the therapeutic efficacy of concentrated nucleated stem cells of the bone marrow obtained by using Harvest's technique in the treatment of critical ischaemia in patients with chronic lower limb arteriopathy. This is gradually and progressively leading to necrosis.
There are several "pros" in using Harvest's system:
- The volume of aspirate is reduced to 240 ml;
- Aspirate is taken with sedation of the patient;
- The procedure lasts 15 minutes and is performed in the operating theatre;
- There is much lower risk of anaemia for the patient;
- There is less risk of contamination;
- There is more cell vitality.
 The cellular composition obtained with Harvest's system differs from the one of Ficoll's. Cell composition of Ficoll's technique mainly contains mononucleated cells (lymphocytes, erythroblasts and monocytes) and few granulocytes. It is important to notice that not only with the two systems there is a change of mononucleated cell content but also the cell composition itself. In Ficoll's system, for instance, pro-erythroblasts and pro-myeloblasts are poorly represented. We must also remember that mesenchymal stem cells are capable of producing CFU and to migrate, as a response to SDF-1. Such specific qualities are particularly present in erythrocyte suspensions rather than in a mono-cellular suspension extracted from marrow aspirate (fig 1).
The cellular composition obtained with Harvest's system differs from the one of Ficoll's. Cell composition of Ficoll's technique mainly contains mononucleated cells (lymphocytes, erythroblasts and monocytes) and few granulocytes. It is important to notice that not only with the two systems there is a change of mononucleated cell content but also the cell composition itself. In Ficoll's system, for instance, pro-erythroblasts and pro-myeloblasts are poorly represented. We must also remember that mesenchymal stem cells are capable of producing CFU and to migrate, as a response to SDF-1. Such specific qualities are particularly present in erythrocyte suspensions rather than in a mono-cellular suspension extracted from marrow aspirate (fig 1).
This study will consider the hypothesis that the extraction of bone marrow obtained by using Harvest's technology, once injected in the muscles affected by critical ischaemia, allows the development of a collateral circle which leads to new-vascularisation: in this way we save the limb and avoid amputation.
In order to evaluate this hypothesis, we enroll 40 patients for a prospective randomized study to be held in the Department of cardiovascular diseases of the research and formation Center, High technology of biomedical sciences GIOVANNI PAOLO II, Catholic University, Campobasso, Italy.

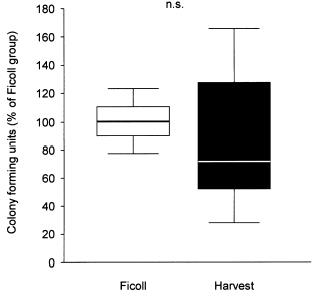
Grafici: Colony-forming activity of isolated BMC using two different protocols (A). Data are presented as percent of colonies observed in the Ficoll group. Transmigratory activity of isolated BMC towards SDF-1 (100 ng/ml) using a modified Boyden chamber assay coated with MatrigelTM (B). Data are presented as percent of cells migrated in the Ficoll group.
Concentration of Bone Marrow Total Nucleated Cells by a Point-of-Care Device Provides a High Yield and Preserves Their Functional Activity Patrick C. Hermann,* et.al Cell Transplantation, Vol. 16, pp. 1059–1069, 2008
We used, for this clinical study, the Smart PR e Pr2 Bone marrow aspirate concentrate system , made by a micro-processor, by a decantation centrifuge, by a supplementary kit, whose sale is presently authorized for the intra-operatory preparation of concentrated cells extracted by the bone marrow of the patient itself and for the preparation of concentrated autologous platelets from the peripheral blood. (FiG.1).
Objective of the study and its end point
We want to establish the therapeutic efficacy of stem cells derived from bone marrow, by using Harvest's technique, in the treatment of lower limb critical ischaemia in patients not eligible for intravascular or surgical revascularisation. The primary end point is to reduce the number of major amputations, above the knee, in patients with critical ischaemia and no more eligible for classic surgery or intravascular procedures. Major amputation, in fact, has a much higher mortality and morbidity, especially under the cardiological point of view, compared to minor amputation .It implies also severe socio-economical and psychological consequences. The loss of a limb creates a great trauma to the patient because it means losing an important part of the self, and this is difficult to accept. Moreover, under the socio-economic point of view, losing a limb often means losing job and almost always a forced retirement, with great lowering of the monthly income. We cannot forget also the costs paid by the Health System (crutches,wheel chair, prosthesis) and all the costs of disability.For all these reasons, amongst secondary end points, surgeons tend to lower the level of amputation as much as possible. This reduces mortality and morbidity, but also allows a better implantation of prosthesis in case of amputation below the knee, or even no use of prosthesis in cases of trans-metatarsal amputation. Moreover, amelioration of ABI, of trans-cutaneous oximetry and collateral circulation are fundamental elements in order to delay a possible amputation and increasing walking capacity in the future. Following informed consent given by the patient, according to the schedule and form proposed by the Ethic Committee of Catholic University for the treatment of stem cells, patients who present inclusion criteria are enrolled in this prospective observational study. Objective efficacy of the treatment will be evaluated on the basis of clinical and instrumental evidence and the survival rate with no need, for the patient, to undergo major amputation. Under the subjective point of view , pain referred by the patient and life quality will be evaluated.
Matherials and methods
We chose 9 patients, 5 women and 4 men, with an average age of 66.7 (54-85 yrs), all of them affected by critical lower limb ischqemia and who already underwent attempts of surgical revascularisation (by-pass or peripheral angioplasty) which were not successful and not suitable for any surgery of intravascular revascularisation. (Fig 2) .These patients underwent echocolordoppler of the lower limbs with ABI measuring, measurement of TcPO2 of the upper side of the foot, angio-CT, angio MRI or lower limb, arteriography in the last 3 months prior to the treatment, assessment of cardiovascular risk, on the basis of factors such as: diabetes mellitus, (fast blood sugar level and glycated Haemoglobin), cigarette smoking, high blood pressure, blood lipids, RCP ( blood results must be no more than 2 weeks old before the treatment). In some cases, in order to have a better evaluation of the perfusion in the lower limbs, we performed a lower limb perfusional scintigraphy using Tetraphosmine , both before and after implant. This was done to obtain a better quantification of the increase of blood flow in the lower limbs.

Surgical treatment
Once anaesthesia (or sedation) was given, we extracted 240 ml of bone marrow by the iliac crest and then it was treated, by using Harvest device (fig. 3) A sample was sent to the laboratory of haematology for the assay of the content and the evaluation of the cell function.

Before giving the injection of concentrated stem cells, we focused our attention on the ischaemic area and the area that we wanted to restore. We marked on the patient's leg the sites of injection (fig 4). Injections must be given at a distance of 1-2 cm from each other , preferably within 1 cm from the vascular bed that we intended to treat, for a global length of 40-80 cm and they can be done with the help of ultrasound scanner.
The volume of each injection (of concentrated cells or placebo) is 1 ml (using a 21 G , length2-4 cm , and for the feet 0.5-1 cm ). The sites of injection are then covered by sterile gauzes and the leg is also wrapped by sterile gauzes.

After the operation, we gave antibiotic prophylaxis with 1 gr Cefazoline within 1 hour from the injection (in case of allergy to penicillin, we administered Vancomicine 1 gr or Clindamicine 600 mg).
Follow up
All treated patients have been and will be followed up after the therapeutic administration after 1 week,1, 3 ,6 and 12 months using arterial echocolordoppler of lower limbs with ABI measurement, measuring TcPO2, subjective evaluation of pain and life quality. Angio-MRI or angio-CT of the aorta and lower limbs, which will be performed 3 and 6 months later, and angiography of the lower limbs after 6 months. Patients who underwent lower limbs perfusional scintigraphy( (before implant) have received another scintigraphy after 3 months, with measurement of the increase of peripheral blood flow. The present follow up seems to be shorter compared to an average follow up of days( range 7-180); this is why results can be premature.
Premature results
No death occurred after 30 days and during the follow up at 6 months. Only 1 patient showed ,5 days after the operation, a clinical picture of acute pulmonary oedema that required urgent admission to intensive therapy with ventilation support from which the patient was progressively weaned. Patient have been followed up at 3 months with measurement of ABI, Tp02, angio- MRI of the aorta and lower limbs and in 3 cases with perfusional scintigraphy with tetrafosfine (those patients who had done a test prior to the operation).
A control angiography was performed just in 1 case with a follow up at 6 months , which showed a marked improvement in the peripheral vascularisation. All these 9 patients had been referred to other hospitals by several surgeons to be amputated at thigh level. Actually, none of them underwent major amputation. The 3 patients who did it, already had necrotic damage when they came to our observation; 2 patients showed necrotic lesions of the fingers and only 1 patient in the third medium of the leg.

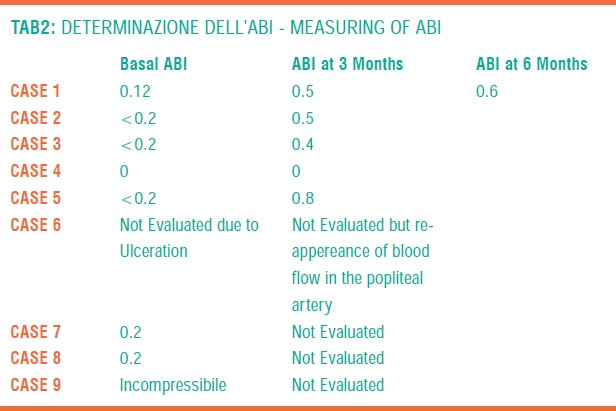


Surely, among other considered parameters, clinical symptoms, ABI and TpO2 give the most significant information in order to evaluate the efficacy of the treatment (TAB 1,2,3).
Apparently, the obtained results seem to be strictly related with cellularity of the explant (TAB 4).The cellular content of Harvest component is characterized by receptorial assay as follows: CD34+ and CD34-, CD 133-,CD 133-, CD 133 VEGF, CD 34 VEGF.
Discussion
The presence of critical ischaemia which is not possible to treat with surgery, revascularisation or even intravascular procedures, makes the patient eligible only for amputation. The use of autologous stem cells extracted by his own bone marrow and injected, during the operation itself, after being purified , in muscles affected by arterial obstructive ischaemia, can be a valid option in these cases. In fact, there is no added risks for anaesthesia, since the operation is performed using local anaesthesia and mild sedation, and no risks of infection since we use the patient's own stem cells injected in the muscles and extracted by his bone marrow through the puncture of the iliac crest. We can describe our results, even if they are referred to a small number of patients. First of all, the clinical response is related to the cellularity of explanted marrow. The higher the cell number, the quicker the symptoms will disappear (rest pain). Secondly, all patients had an increase of ABI and Tpc02. The presence of a limited sample allows to say that such an increase is statistically significant. Thirdly, major amputation was predicted in 100% of cases, namely all 9 patients. Only 3 patients, 35%, underwent amputation but showed necrotic lesions from the moment of enrolment in the study. Thus, we can state that about 65% of treated patients (6 patients) have been saved from leg amputation. Moreover, the 3 patients have been amputated below the predicted level, namely the thigh, and all of them are diabetic. In fact, the presence of diabetes enhances both suprainfections of soft tissues underlying necrotic areas and osteomyelitis. The quick clinical response within the first 24 hours after the implantation with disappearance or important reduction of rest pain, of hypothermia, seems to be due, in our opinion, to the action of cytokines and tissue modulators released by stem cells in the area of peripheral ischaemia.


Conclusions
We are convinced that the small number of studied patients and the duration of follow up do not allow us to draw definitive conclusions, even if premature results are very promising and important. We then need to keep treating these patients with critical ischaemia, not eligible for revascularisation, and try to be very accurate in the selection of patients by creating inclusion and exclusion criteria, without extending this kind of approach to patients with "dry" widespread necrosis or with acute depasse' ischaemia, or, even worse, with clinical pictures of critical ischaemia evolving to "wet" gangrene, as we often see in diabetic patients.
Modugno P., De Filippo CM, Caradonna E., Centritto E.M., Amatuzio M., F. Alessandrini.
Bibliografia
1. R. Nowygrod, N. Egorova, G. Greco, P. Anderson, et all.: Trends, complications, and mortality in peripheral vascular Surgery. J Vasc. Surgery 2006; 43: 205-16
2. Clar DG, Dayal R, Faries PL, Bernheim J, Nowygrod R, Lantis JC, Beavers FP, Kent KC. Tibial angioplasty as an alternative strategy in patients with limb threating ischemia. Ann Vasc Surg; 2005 Jan; 1: 63-68
3. M.R. Nehler, Brass E.P., R Anthony; J. Dormandy et all: Adjuntive parenteral therapy with lipo-ecraprost a prostaglandin E1 analog, in patient with critical limb ischemia undergoing distal revascularization does not improve 6-months outcomes
4. Dormandy J, Heeck L, Vig S. The natural history of claudication: risk to life and limb. Semin Vasc Surg. 1999 Jun;12(2):123-37
5. German Diabetes Research Institute. Diabetes mellitus in Germany − Data situation and risk profile, 2003. http://www.diabetes.uniduesseldorf.de/download/Fakten_ zum_Diabetes [facts on diabetes].pdf.
6. Lawall, H. Peripheral arterial occlusive disease: diagnosis and treatment in diabetes mellitus. Cardiovasc. 2;238-43, 2002
7. Isner JM, Asahara T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J. Clin Inves 1999; 103: 1231-12365
8. Crosby JR, Kaminski WE, Schatteman G, Martin PJ, Raines EW, Seifert RA, Bowen-Pope DF. Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation; Clin Res. 2000; 87:728-739
9. Hristov M, Erl W, Weber PC. Endothelial Progenitor Cells: Mobilization, Differentiation, and Homing . Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23:1185-1188
10. Iwasaki H, Kawamoto A, Ishikawa M, Oyamada A, Nakamori S, Nishimura H, Sadamoto K, Horii M, Matsumoto T, Murasawa S, Shibata T, Suehiro S, Asahara Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation. 2006; 113: 1311-1325
11. Asahara T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circulation Research. 1999; 85: 221-228
12. Caplice NM, Doyle B. Vascular progenitor cells: origin and mechanisms of mobilization, differentiation, integration, and vasculogenesis. Stem Cells Dev. 2005; 14: 122-39
13. Bauer SM, Goldstein LJ, Bauer RJ, Chen HY, Putt M, Velazquez OC. The bone marrow-derived endothelial progenitor cell response is impaired in delayed wound healing from ischemia. J of Vasc Surg. 2006; 43: 134-141
14. Jia L, Takahashi M,Yoshioka T, Morimoto H, Ise H, Uiehi I. Therapeutic potential of endothelial progenitor cells for cardiaovascular diseases. Current Vascular Pharmacology. 2006; 4: 59-65
15. Tareishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, Shimada K, Iwasaka T, Imaizumi T, Therapeutic angiogenesis for patients with limb ischemia by autologous transplantation of bone-marrow cells: a pilot study and a randomized controlled trial. The Lancet; 2002; 360: 427-435
16. Seiler C, Pohl T, Wustmann K. Hutter D, Nicolet PA. Promotion of collateral growth by granulocyte-macrophage colony-stimulating factor in patients with coronary artery disease: a randomized, double blind, placebo-controlled study; Circ 2001; 104: 2012-2017
17. Kamihata H, Matsubara H, Nishiue T, Fujiyama S, tsutsumi Y, Ozone R, Masaki H, Mori Y, Iba O, Tateishi E, Kosaki A, Shintani S, Murohara T, Imaizumi T, Iwasaka T. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligans, and cytokines. Circ 2001;104:1046-1052
18. Kamihata H, Matsubara H, Nishiue T, Fujiyama S, tsutsumi Y, Ozone R, Masaki H, Mori Y, Iba O, Tateishi E, Kosaki A, Shintani S, Murohara T, Imaizumi T, Iwasaka T. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligans, and cytokines. Circ 2001;104:1046-1052
19. Shintani S, Murohara T, Ikeda H, Ueno T, Sasaki K, Duan J, Imaizumi T. Augmentation of postnatal neovascularization with autologous bone marrow transplantation, Circ. 2001; 103:897-895
20. Ikenaga S, Hamano K, Nishada M, Kobayashi T, Li T, Kobayashi S, Matsuzaki M, Zempo N, Esato K Autologous bone marrow implantation induced angiogenesis and improved deteriorated exercise capacity in a rat ischemic hind limb model. J Surg Res; 2001; 96: 277-283
21. Yoshida M, Horimoto H, Mieno S, Nomura Y, Okawa H, Nakahara K, Sasaki S. Intra-arterial bone marrow cell transplantation induces angiogenesis in rat hindlimb ischemia. Eur Surg Res 2003; 35: 86-91
22. Iba O, Matsubara H, Nozawa Y, Fujiyama S, Amano K, Mori Y, Kojima H, Iwasaka T. Angiogenesis by implantation of peripheral blood mononuclear cells and platelets into ischemic limbs 2002; 106: 2019-2025
23. Esato K, Hamano K, Li TS, Furutani A, Seyama A, Takenaka H, Zempo N. Neovascularization induced by autologous bone marrow cell implantation in peripheral arterial disease. Cell Transplant 2002;11(8):747-752
24. Saigawa T, Kato K, Ozawa T, Toba K, Makiyama Y, Minagawa S, Hashimoto S, Furukawa T, Nakamura Y, Hanawa H, Kodama M, Yoshimura N, Fujiwara H, Namura O, Sogawa M, Hayashi J, Aizawa Y. Clinical Application of bone marrow implantation in patients with arteriosclerosis obliterans and the association between efficacy and the number of implanted cells. Circ J. 2004; 68: 1189-1193
25. Heeschen C, Hamm CW, Mitrovic V., Lantelme NH, White HD: Platelet Receptor Inhibitionb in Ischemic Syndrome Management ( PRISM ) Investigators. Circulation 2004 Nov 16; 110(20): 3206-12
26. Kawamura A, Horie T, Tsuda I, Ikeda A, Egawa H, Imamura E, Iida J, Sakata H, Tamaki T, Kukita K, Meguro J, Yonekawa M, Kasai M. Prevention of limb amputation in patients with limb ulcers by autologous peripheral blood mononuclear cell implantation Therapeutic Apheresis and Dialysis; 2005; 9:59-63
27. Kisucka J, Butterfield CE, Duda DG, Eichenberger SC, Saffaripour S, Ware J, Ruggeri ZM, Jain RK, Folkman J, Wagner DD. Platelets and platelet adhesion support angiogenesis while preventing excessive hemorrhage. Proceedings of the National Academy of Sciences January 24, 2006; 103:855-860
28. Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003 Feb 13;348(7):593-600



