from Leadership Medica n. 10/2005
Abstract
The mortality rate due to AIDS has gradually dropped in the past 9 years. This fact is mainly attributed to antiretroviral treatment. We shall hence study the related evidence, which has been found to be highly deficient. The main reasons lie in some less known statistical repercussions and in the abnormal setting of drug efficacy studies, which in the first place are designed to obtain results on "surrogate markers" rather than on clinical advantages. Other options could lead both to progress in treatment and to a better understanding of this complex and articulated syndrome.
Index
- Introduction
- 1. HISTORY OF ANTIRETROVIRAL TREATMENT
- Evaluation of data related to the current progressive reduction in mortality since 1996
- The treatment's risk-benefit evaluation - HAART-related toxicity
- 2. SURROGATE MARKERS' VALIDITY
- 3. PROPOSALS FOR ALTERNATIVE TREATMENT
Introduction
 Starting from the assumption that every cognitive progress' foundation is a critical awareness of previous knowledge with the recognition of past errors, to this end we propose: 1) some methodological considerations on antiretroviral treatment in the light of the results gradually obtained; 2) a discussion on the treatment's current goals (control of surrogate markers); and, 3) an evaluation of practical alternative proposals.
Starting from the assumption that every cognitive progress' foundation is a critical awareness of previous knowledge with the recognition of past errors, to this end we propose: 1) some methodological considerations on antiretroviral treatment in the light of the results gradually obtained; 2) a discussion on the treatment's current goals (control of surrogate markers); and, 3) an evaluation of practical alternative proposals.
What appears clear is that antiretroviral treatment has undergone important modifications through the years and that a remarkable reduction in AIDS-related mortality has been observed since 1996, the year new drug classes were introduced. However the assumption that such a reduction results only from this factor is very limiting. This review plans on demonstrating it in three short chapters:
1) HISTORY OF ANTIRETROVIRAL TREATMENT
Evaluation of the mortality rate reduction Evaluation of the treatment's risks and advantages
2) VALIDITY OF SURROGATE MARKERS
3) PROPOSALS FOR ALTERNATIVE TREATMENT
1. HISTORY OF ANTIRETROVIRAL TREATMENT
Thousands of studies, which tested the efficacy of treatment in HIV infections, have been published. However it is unusual that those which are considered as methodologically appropriate are rare and have invariably produced irrelevant or negative results for antiretroviral drugs.
We should specify right away that a correct evaluation of a drug's efficacy necessarily requires detailed preliminary studies followed by methodologically appropriate clinical studies (randomized, placebo controlled, double blind) to reduce any bias. We wish to recall that a drug against AIDS (ditiocarb), which seemed to be promising and which had positively passed various small well structured studies, was withdrawn from circulation due to just one negative study (1). This study was more extensive, had a greater statistical relevance and was on the other hand interrupted at an early stage . Such a measure is justified as long as the premises are correct because 100 positive corroboration controls are not worth a good falsification, as Karl Popper the philosopher of science taught us. However in other similar situations the same principle was not applied. We must also observe that currently:
1) to enable speedy approval of drugs we resort to fast track approval, which drastically cuts short the preliminary phases of experimentation and study to 24 weeks (2) (normally 5-10 years);
2) clinical trials with appropriate features are rare and they do not show advantages for treated patients (S. Garattini (3)); and,
3) from 1993 these were curiously abolished for "ethical reasons", in other words not to deprive some patients (those meant for placebo treatment in the control group) of the benefits of the antiretroviral drugs studied. But these benefits are only presumed and not proved at all. We can explain this by merely mentioning the results of some studies, which reveal the periods studied and which can be considered as paradigmatic by the community of researchers.
A. (1987-1990) Evidence of AZT inefficacy for individuals suffering from AIDS.
The first antiretroviral drug (AZT) was approved following a study - published in 1987 and closed prematurely 4 months after the start, which proved that the mortality rate in patients suffering from AIDS who had received a placebo treatment seemed to be far higher than those treated with AZT (19 vs. 1) (4); however this study was proved to be both fraudulent (5) and highly mistaken in the evaluation of results (6). To confirm the lack of efficacy, a follow up of the same group of patients performed by the same researchers revealed a high mortality rate after a few months (42.4% after 21 months) and a lower mortality rate for the group that had taken ACT for a shorter period (35% in the same period) (7,8).
B. Evidence of the inefficacy of AZT in AIDS prophylaxis in asymptomatic individuals (1990-1995).
In the study that in 1990 inaugurated the use of AZT in asymptomatic seropositive individuals (Volberding) (9), the best results declared in terms of survival and quality of life were reviewed by the same author: 4 years later: in 1994 he admitted that "when life threatening side effects were also counted the placebo group in practice had gained some advantage on both AZT groups in terms of interval without symptoms of disease or toxicity" (10). This statement should have been handed over to public opinion much earlier.
Still another study, the last in sequential order, had a placebo group: the Concorde Trial (which designed to calm doubts and controversy instead gave rise to prolonged discussions). A clear disadvantage was proved in the group of patients who had been administered early treatment with AZT (25% additional deaths though partly due to causes other than AIDS) (11). But this proof did not involve an adjustment in the therapeutic trend, rather the opposite.
BOX 1 The apparently illogical consequence of these disappointing results was that almost the entire world of research grouped round the same previous setting without considering the data available. At that stage control groups treated with placebo were not used anymore in experiments and the new drugs (inserted in the "life saving" category) were and still are assessed through a comparative study with the previous ones in an endless logical chain, whose first weak link was to be precise the (in) efficacy of AZT. But there is another consequence that is less serious - real progress was obscured by the lack of methodology in the premises.
C. (1995-2003) David Ho's revolutionary discovery. The multidrug treatment.
The turning point was marked by an article authored by David Ho and published in the New England Journal of Medicine (12) in 1995. In this article the American researcher declared with conviction that the time had come to forcefully strike HIV at an early stage with many drugs, … because monotherapy was heading for failure (an explicit authoritative admission).
Three "scientific pillars" supported his theory:
1) the sudden detection of large quantities of virus with the new tests (quantitative PCR, which would indicate the Viral Load);
2) a new study in which AZT treatment administered in the early stage of the infection seemed to produce a considerable advantage compared to untreated patients (13);
3) the availability of other drug classes, in other words protease inhibitors (three of these were approved by the FDA in less than three months, which was no doubt a record time) (14).
Concerning the first point the use of the modified PCR method to calculate the Viral Load was considered as invalid and a real oxymoron (15) by a leading expert who was also the one to discover it. He was the Nobel prize winner Kari Mullis. However, whether the viral load found was high or low, the infected lymphocytes, which were very few before 1995 remained very few (1:1000, 1:10.000) in the following years though they were surrounded by millions of "viral particles" (16). Concerning the second point, the study mentioned by D. Ho (17) would prove a paradoxical result from a virological perspective, in other words a clinical advantage for patients and at the same time void of effects on the disease's causal agent. In fact the duration and characteristics of the initial mononucleosis syndrome at the moment of maximum viral replication at the start of the immune response was the same both in those who had taken AZT and in those who had taken the placebo. The same occurred concerning the isolation of the surrogate P24 marker and the RNA particles revealed by PCR (with no significant difference between the two groups).
The study however lasted for a few initial months compared to the disease, whose average estimate incubation period was 10-14 years.
The third point, in other words the possibility of considerably limiting the infection from spreading by treating it at an early stage, was a mere theory, an assumption to be checked though it was presented as a solution that seemed quite obvious.
This sufficed to once again speed up the huge convoy of HAART (Highly Active Antiretroviral Therapy) prophylaxis and treatment for all seropositive patients for an unlimited period of time.
This convoy was running the risk of coming to a standstill with the sole use of AZT.
The initial success was so considerable that the expression "Lazarus effect" (18) was coined to designate the fact that hospital wards had been emptied because patients had recorded such an improvement and did not require frequent hospitalization anymore.
In many centres more or less all patients were treated with HAART. In three years - from 1994 to 1997 - the European percentage of untreated patients dropped from 37% to 9%. The number of AIDS cases dropped considerably and so did the incidence of opportunistic diseases (i.e. CMV retinitis, Cryptosporidiosis, cerebral toxoplasmosis). But in the years that followed problems of multiple toxicity related to "lifelong therapy" appeared in all their clarity.
The initial enthusiasm was partly put back into perspective (it was not "strike hard, strike early" anymore) and current international protocols are far more restrictive in recommending the start of treatment. This is an admission that the course so enthusiastically indicated by D. Ho was not sustainable.
Evaluation of data related to the current progressive reduction in mortality since 1996.
There is doubtless a considerable reduction in the mortality of individuals suffering from AIDS after 1996, the year "cocktails" (combinations of three drugs) were introduced, in other words in the subgroup of seropositive individuals who had already contracted an opportunistic disease. The explanation is however not easy to grasp as the information is "unrefined" and comprises many confusing variables and factors (i.e. it combines both treated and untreated patients, those who cooperated with the physician's specifications and those who did not, those who followed other treatment and those who followed a prophylactic treatment).
Edges are very poorly defined in murky waters and everyone believes they see the profile they wish to see. In other words the phenomenon's explanation is hindered by the lack of a crucial comparison between treated and untreated groups with a correct degree of randomization. For example in two of the studies that are most frequently mentioned to support this thesis (clear reduction in the mortality rate compared to the past), the control group that was not administered the placebo was either insufficient, inadequate or absent; besides most individuals involved in the studies were initially asymptomatic (let us recall that the mean incubation period for untreated AIDS is 10-14 years, which corresponds to 5% - 3.6% of its yearly incidence).
In the study conducted by Palella (20) the authors found that the mortality in asymptomatic seropositive individuals (with less than 100 CD4) treated with antiretroviral drugs was 8.8/100 people a year. The mortality rate proved to be less after the introduction of protease inhibitors.
In the second follow up study conducted on 1,219 seropositive individuals (87% were initially asymptomatic) treated with antiretroviral drugs, the mortality rate dropped to 6.7% a year with a clear reduction in the introduction of Protease Inhibitors (PI) after the year 1996. But the very authors also made an embarrassing confession: patients who had taken protease inhibitors had a double risk of dying (though a multivariate analysis showed that their use did not alter the outcome) (21). Clearly these results were not as expected!
BOX 2 The United States' definition of AIDS differs from the European one and both were introduced in 1993: the former also includes asymptomatic individuals with CD4 counts that are below 200/µL. This can explain the lesser mortality rate considering that over 60% of AIDS cases reported every year in the US belongs to this subgroup (I). In Europe and in Italy asymptomatic individuals are excluded from our definition of AIDS. In Italy however epidemiological data confirms a clear reduction in the death rate among seropositive individuals who were already infected by AIDS.
 Fig.1 The plot shows how in Italy the mortality rate of individuals suffering from the syndrome after 1-2-3-4 years following the initial diagnosis dropped from 1988 to 2004 (i.e. in 1988 the mortality rate 3 years after the diagnosis was 78% while in 2004 it dropped to about 16%).
Fig.1 The plot shows how in Italy the mortality rate of individuals suffering from the syndrome after 1-2-3-4 years following the initial diagnosis dropped from 1988 to 2004 (i.e. in 1988 the mortality rate 3 years after the diagnosis was 78% while in 2004 it dropped to about 16%).
This greater survival rate was entirely attributed to the use of drug cocktails; however other factors too are involved as possible causes of these changes in time:
a) A new selection of patient types compared to the past with a considerable reduction in the quota of drug addicts who are struck by a greater mortality rate (in Italy the quota of drug addicts has dropped from 70 to 20% (22)). This was confirmed by the EuroSIDA study, which noticed "a significant reduction in the time of death in patients who were not treated" (23) (this further proves how the comparison with what occurred in the past can be misleading in this context).
b) A transitory beneficial effect of one or more components of the cocktail (this point will be developed in the following chapter) irrespective of the antiretroviral treatment (24).
c) HAART's protective antibiotic effect towards opportunistic pathogens (25).
d) Improved specialist prophylactic, diagnostic and therapeutic skills.
e) The exclusion of the death count resulting from antiretroviral drugs in seropositive individuals free of AIDS (this plot only counts individuals with AIDS).
BOX 3 Two are the queries, whose clear answers would be useful: how many are the seropositive individuals who have not been infected by AIDS thanks to drugs and who have lived discretely well? How many instead are those who have long remained asymptomatic without HAART treatment and who instead have suffered from heavy side effects? We lack this crucial information to draft a final balance, hence we can only make some assumptions on the basis of indirect data with the limits this process involves.
Since many patients have benefited from drugs, we wonder whether it is possible to isolate the factors that are behind their improvement, reducing at the same time the highly relevant pointless and harmful factors.
The treatment's risk-benefit evaluation - HAART-related toxicity
Drugs must only be offered when there is a strong sign of their advantages especially when the risk is very clear (D. Sackett, the "father" of Evidence-Based Medicine) (26).
Regarding antiretroviral drugs it is clear that they were able to solve clinical situations that would have been desperate once.
They have succeeded in stabilizing discrete conditions for a protracted period of time in the type of patient who was once destined to fall ill repeatedly. The reduction in opportunistic diseases was drastic.
But a more objective perspective requires an objective performance appraisal both in the short term and in the medium and long term too. Hence we shall study:
A) declared toxicity,
B) HAART's effects on seronegative individuals,
C) the EuroSIDA study,
D) the American study on 4th degree adverse events, and,
E) long term non progressors.
A) Declared toxicity
Adverse events envisaged and listed in the information sheets are many. They strike patients in a high percentage of cases appearing either at an early stage or more often at a later stage. They have a moderate (i.e. nausea) and high intensity with a risk for life (i.e. lactic acidosis), besides they affect the gastrointestinal system (i.e. diarrhoea), the haemopoietic system (i.e. anaemia), the nervous system (i.e. neuritis) and the skin (severe allergies). They can cause lipodistrophy and progressive liver failure.
B) HAART administered to "non infected" individuals
Warnings concerning the drugs in question also specify that toxic symptoms can be confused with those of HIV. To avoid this risk, nothing is better then to check them on healthy uninfected individuals.
The occasion is provided by work accidents and the related post-exposure statistics. In the Italian ones for example (27) it is clear that: "63.2-66.5% of cases complained of side effects; treatment was discontinued due to them in 28.7-32% cases after 7-8 days (average)".
They were healthy individuals who had to be treated for a month! No difference between the various protocols occurred in the proportions of workers who discontinued treatment.
C) The EuroSIDA Study: "negligible" HAART-related toxicity.
It would be very strange then to observe that these side effects, which are so expected and frequent in healthy individuals, should after all be negligible in seropositive individuals.
And yet this very fact ensured the success of an extensive multicentre prospective study (EuroSIDA study) (28) involving 5 large groups of seropositive individuals: a clear reduction in the mortality rate and incidence of AIDS (up to one fifteenth compared to the pre-HAART period) (29) was detected from 1995 to 2002. Results seem to be reassuring also because "problems related to serious adverse events […] did not alter the population's mortality and morbidity". The difference between total mortality and HIV-related mortality was in fact very small. It is curious to observe that in another section the authors of the same study say that, considering the 23% reduction in AIDS-related yearly mortality rate, there is a "32% yearly increase in other causes of death" (the term "other" mainly designates HAART-related side effects). However: a) a considerable group of patients had CD4 over 200 (many > 500) and were initially asymptomatic, b) even those who had taken drugs for a few days were considered as "treated" for the study's entire duration, d) we do not know how many have taken them for the entire observation period, how many have discontinued them and for how long they have discontinued their use, e) there is no control group, hence we cannot understand how a statistical method such as the Intention to Treat Approach, which clearly envisages the presence of a control group, has been implemented, f) calculations do not include the negligible group of those who dropped out during the followup phase (30).
We believe that it is a very complex analysis, which gives rise to more questions and doubts than the ones it solves.
D) Grade 4 adverse events.
A clearer contribution towards understanding the various factors involved is given by a study conducted on over 2.000 seropositive patients (60% of whom were initially asymptomatic) treated with HAART (31). Details were published in JAIDS in 2003. The incidence of serious adverse reactions was double the opportunistic diseases (675 vs. 332); in the same manner there were more deaths caused by toxicity-related events than those resulting from opportunistic diseases (153 vs. 117).
Mortality in case of an adverse event was high (22.7%). It is important to note that 201 entirely asymptomatic patients with CD4>200 suffered from serious toxicity and 84 of them died due to drugs without having ever suffered from opportunistic diseases.
NB: lastly numbers reported in the study and in the tables are inconsistent.
BOX 4 There are further important considerations to make: a) deaths due to serious toxicity without AIDS cannot be strictly counted among deaths caused by AIDS. Hence the need to keep in mind that in mortality rate plots: a) there are dead who do not appear; b) it is easy for patients who died of toxicity to be counted among deaths caused by AIDS if they had previously suffered from an opportunistic disease (individuals with AIDS who have died due to iatrogenic causes); c) the study clearly revealed that the longer a patient had taken drugs the greater probability he had of suffering from serious toxicity (30.8% after 36 months) with a clear progression in time.
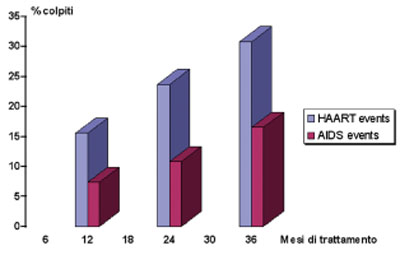 Fig.2 Probability of AIDS or adverse events in treatises (Reisler RB et al. JAIDS 2003;34:379-86)
Fig.2 Probability of AIDS or adverse events in treatises (Reisler RB et al. JAIDS 2003;34:379-86)
The probability of a serious adverse event resulting from HAART is about double that of an opportunistic infection with a considerable increase in time (according to the study the rate of mortality would be similar in both cases).
Calculating the statistics of mortality caused by AIDS with data provided by the study in question, the mortality rate is higher due to treatment than to diseases in a period of 20.7 months (average) (as per the table below).

E) Long Term Non Progressors
Another observation that is worthy of note is that invariably all LTNP (long term healthy seropositive individuals - Long term non progressors) reported in literature in the period prior to 1995 (32) and more recently up to 2004 (33) had not taken antiretroviral drugs.
Conclusions
Though it is likely an associated positive contribution to new treatment, caution is a must due to the incident of ATZ treatment, which was considered valid in the past but proved to be disadvantageous in time (D. Sackett). At a time when all researchers seem to support and carefully use "evidence-based medicine", evidence is lacking in this case and remains unsought. At times the less presentable aspects are also expertly "toned down", as we observed in the EuroSida study.
2. SURROGATE MARKERS' VALIDITY
Come riferito nel precedente paragrafo, gli studi clinici randomizzati controllati con placebo hanno portato a risultati complessivamente negativi e dal 1992 non ne sono stati più iniziati altri. Inoltre gli obiettivi (end points) clinici sono stati sostituiti da obiettivi laboratoristici, (i “marker surrogati”), che avrebbero dovuto rispecchiarli fedelmente.
BOX 5 The optimal surrogate marker's features
The surrogate marker must have the following features to be practically useful: a) a clear, logical, pathophysiological association with the disease, b) a well defined role in the natural history of the disease, c) it must be detected in most affected individuals, d) it must change with the clinical condition in a measurable manner (both in the disease's progression and regression), e) it must change consistently with the success or inefficacy of the therapeutic treatment.
Viral Load and Other Markers
Efficacy studies conducted on new drug combinations hence focus on extremely clear viral suppression. The advert for a recently introduced drug (enfuvirtide) launches the following message: "Detectable is unacceptable" stressing on resetting the viral load back to zero. In other words, despite its clear inadequacy, the treatment's first target has been the same for the past 10 years.
The viral load has often proved to be a poorly relevant index both concerning the immune system's degree of competence and the clinical evolution. In studies focused on it the divergence between CD4 and the viral load, in other words the increase in CD4 despite the lack of a reduction in the viral load, occurs in a relevant number of cases (36 ). A group of researchers declared the following on the topic: "These observations also give rise to doubts concerning emphasis on HIV RNA levels as a surrogate marker in clinical trials." (37)
BOX 6 Reported below are some of the many observations published in literature.
In one of the studies mentioned (II), "T lymphocytes CD4(+) and CD8(+) (including the memory and progenitor subpopulations) had increased in a similar manner among patients with plasma viral loads that either persistently dropped or temporarily dropped or did not diminish at all." In another study (III) the expected results occurred only in 34% of cases (low viral load and increased CD4 levels) and in 9% of cases (high viral load and CD4 reduction).
Paradoxically the viral load is not linked to the percentage of infected cells, hence according to recent publications the number of infected CD4 lymphocytes is limited to 1:1000, 1:10.000 (IV) and the reduction in the various lymphocyte populations (numbering CD8 lymphocytes) is necessarily attributed to processes other than direct viral cell killing (V). Recent publications confirm that direct cell killing in HIV cannot explain the reduction in lymphocytes and progenitor cells(VI).
The progenitor cells that however have a reduced regenerative capacity are not infected. (VII)
According to other studies high viraemia and low immunoactivation are associated with a poor cell apoptosis (VIII) and, on the contrary, chronic immunoactivation even when it is associated with viraemia control leads to clinical progression. (IX)
We can conclude that the VL is not a very reliable marker and that it is erratic because unforeseen and inconsistent results greatly surpass 4-6% (a tolerable gap). If clinical and immunological improvements can occur despite the VL, then it would be logical that its use should be discontinued in the drug approval process for "HIVrelated diseases" and that we should refer to other more significant parameters.
Probably there are no excellent markers, hence it is imperative to however maintain the reference to clinical endpoints such as the state of well-being, the quality of life, the incidence of diseases, side effects and mortality (38).The following seem to be better markers than the VL:
a) The CD4 count in the blood (39), the increase or reduction in the state of chronic immunoactivation (40) along with the excessive production of certain cytokines (41), the Th1-Th2 shift (42), cell health indicators and the redox status (43).
We must keep in mind that the very CD4 count is not perfectly related to the immune system's efficiency, for example opportunistic diseases can also strike individuals with CD4 >200 (cerebral toxoplasmosis strikes 9% and PCP 14.5% of individuals with over 200 CD4, even with pre-AIDS antiretroviral treatment - Italian data from 1999 to 31.12.04, AIDS Operational Centre), while some individuals can remain asymptomatic for long with CD4 < 100.
The chronic immunoactivation condition is characterised in seropositive individuals who face the risk of a deteriorating condition (44). Chronic immunoactivation leads to clinical progression even when it is associated with viraemia control (45). Stress oxidative markers play a relevant role. Passi observed that oxidative stress was gradually more evident on various parameters with the increased evidence of immunodeficiency (46).
3. PROPOSALS FOR ALTERNATIVE TREATMENT
Immunomodulating Drugs
A. Fauci, director of the American NIAID (47), L. Montagnier (48) and many other researchers have highlighted the usefulness of controlling chronic immunoactivation and oxidative stress in HIV infections by proposing targeted treatments: there are drugs that could control immunoactivation (and indirectly the improvement of immune conditions, the same HIV infection and clinical conditions). These number:
a) Antioxidants: the use of antioxidants has been extensively accepted though we must keep in mind that results have often been disappointing.
This can be explained by the fact that only one antioxidant was used or that inappropriate dosages of substances, which can become prooxidant in the body were administered. Siro Passi's more convincing theory states that an appropriate diet correction associated with a pool of natural antioxidants measured to suit the deficiencies found leads to real benefit concerning the inhibition of the disease's progression. Small controlled studies have proved the integrated diet's good efficacy in reestablishing normal levels of antioxidants (49).
Other interesting and complex theories too lean towards the same direction: those proposed by Eleni Eleopulos Papadopulos (50) and Heinrich Kremer (51).
b) The same antiretroviral drugs seem to have the abovementioned activities (52). Most likely this effect can be obtained with lower dosages and different drug combinations compared to those used according to current protocols designed to achieve control of the VL (53). Anti-HIV drugs have an antiretroviral effect and they also modulate immunoactivation, which is controlled or reduced by them (54).
c) Some antinflammatory drugs influence the control of immunoactivation: low dosage cortisone drugs (55), acetylsalicylic acid (56) and salazopyrin (57).
Similar better targeted drugs with less side effects could be found. (Box 7)
We propose better evaluating the clinical end-points and their association with:
a) immunoactivation parameters;
b) redox conditions and other cell health indicators;
c) the lymphocyte and CD4 count;
d) reactivity to the cell immunity test (Multitest); and,
e) the cytokine dosage.
The use of antiretroviral drugs at minimum effective dosages can be taken into consideration to control the parameters mentioned, but not the VL, in a pattern that envisages discontinuing the treatment when improvements are consolidated. Hence these drugs need not be taken for life.
BOX 7 Summarising, treatment could be scheduled as follows:
1) Recognition and correction of unhealthy lifestyles (problems faced mainly by many dissident scientists numbering P Duesberg, E Eleopulos, J Lauritsen, S Passi, L De Marchi and H Kremer);
2) Implementation of a rich and adequate diet (S Passi);
3) Recognition (cell health indicators) and correction of metabolic deficiencies (S. Passi, H. Kremer);
4) Use of antiretroviral drugs only if there is a decided tendency for conditions and immunity to worsen (CD4 < 200 and important signs of immunoactivation and immunodepression, opportunistic diseases) for limited periods;
5) Concerning antiretroviral drugs, we theorize that less costly combinations (one or two drugs) could be investigated at doses that can control crucial metabolic aspects (i.e. the use in monotherapy of protease inhibitors, whose positive effects in vivo have already been reported (dosages that are 30 times less than those specified in the treatment can increase both peripheral blood cell survival (X) and clinical survival (XI);
6) Reintroduction of reliable study criteria of drugs, including randomised double blind clinical trials with placebo ("vanished" for over 12 years), removing the convenient loopholes of the fast track approval.
Postscript
Both the logic and clinical application of antiretroviral treatment applied to HIV infections was extensively criticised in the past by "dissident" researchers (E Eleopulos P Duesberg), but their argumentative statements were not followed by scientific confutations. However we report them for detailed study purposes. (58).
Fabio Franchi
Specialist in Hygiene, Preventive Medicine
and Infectious Diseases in Trieste, Italy
Note
I HIV/AIDS Surv Rep dec 1996
II Lu W. Et al. Blood 2000, op.cit.
III VL alto CD4 invariati o aumentati
• . Aiko Okano et al. Discordant Movement of CD4-Positive T-Cell Count in HIV-1 Infected Patients with HAART Failure Jpn. J. Infect. Dis., 55, 62-65, 2002 :
IV CD4 infettati= 1:1000 - 1:10.000
• Rosenberg YJ et al. Immunol Today 1998 op.cit.
V Diminuzione CD4: meccanismo diverso dal cell killing Rosenberg YJ et al. Immunol Today 1998; 19:10-7
VI apoptosis cellule non infette
• Silvestri G, Feinberg MB. J. Clin. Invest. 112:821–824 (2003).
• Dianzani U et al. Role of FAS in HIV infection. Curr HIV Res. 2003 Oct;1(4):405-17.
VII Cellule progenitrici non infette
• Rosenzweig M et al. Transduction of CD34+ hematopoietic progenitor cells with an antitat gene protects T-cell and macrophage progeny from AIDS virus infection. J Virol. 1997 Apr;71(4):2740-6.
• Scadden DT et al.Hematopoietic stem cells in HIV disease. J Natl Cancer Inst Monogr. 2001;(28):24-9.
• Weichoold FF et al. Neither HIV-1, nor HIV-2 infects most-primitive human hematopoietic stem cells as assessed in long-term bone marrow coltures. Blood 1998;91:907-15.
VIII High virus, low immune activation, sustained CD4 counts -
• . Douek, D.C., et al.. HIV preferentially infects HIV-specific CD4+ T cells. Nature 2002. 417:95–98)
• Silvestri G, Feinberg MB. Turnover of lymphocytes and conceptual paradigms in HIV infection J. Clin. Invest. 112:821–824 (2003)
• Silvestri G et al. Nonpathogenic SIV infection of sooty mangabeys is characterized by limitedbystander immunopathology despite chronic high-level viremia. Immunity. 2003 Mar;18(3):441-52.
• “The degree of apoptosis correlates with the state of immune activation” “apoptosis concerns all lymphocyte subset”
• Gougeon ML et al.Comparative analysis of apoptosis in HIV-infected humans and chimpanzees:relation with lymphocyte activation. Immunol Lett. 1996 Jun;51(1-2):75-81.
IX Long-term HIV suppression does not invariably lead to immune restoration.
• Nadine G. Pakker*, the INCAS Study Group. Immune restoration does not invariably occur following long-term HIV-1 suppression during antiretroviral therapy. AIDS 1999, 13:203–212.
X Lu W. Blood 2000 Op cit
XI Martin Hirsch et al. for the Protocol 039 Study Group A Randomized, Controlled Trial of Indinavir, Zidovudine, and Lamivudine in Adults with Advanced Human Immunodeficiency Virus Type 1 Infection and Prior Antiretroviral Therapy JID 1999;180:659-65.
G. Pierone et al. Lopinavir/Ritonavir Monotherapy from NNRTI-Based HAART in HIV-Infected Patients With Complete Viral Suppression;
24-Week Interim Analysis. Poster TuPeB4595 Bangkoch AIDS Conference 2004
Bibliografia
1 The HIV87 Study Group. Multicenter, randomized, placebo-controlled study of ditiocarb (Imuthiol) in human immunodeficiency virus-infected asymptomatic and minimally symptomatic patients. AIDS Res Hum Retroviruses. 1993 Jan;9(1):83-9.
2 Pau AK Antiretroviral therapy-associated serious and life-threatening toxicities. Curr Treat Options in Inf Dis 2003; 5:101-12
3 Garattini S. Il punto sulla zidovudina. Ricerca e Pratica; nov - dic 1993;185-191.
4 Fishl M. et al. N Engl J Med 1987; 317 (4): 185-91
5 John Lauritsen, 'Poison by Prescription; The AZT story' Asklepios Press
6 Duesberg P, Koehnlein C and Rasnick D 2003 The chemical bases of the various AIDS epidemics: recreational drugs, anti-viral chemotherapy and malnutrition; J. Biosci. 28 383–412]
7 Fischl, MA, & the AZT Collaborative Working Group Prolonged zidovudine therapy in patients with AIDS and advanced AIDS-related complex. JAMA 1989;262:2405-10.
8 Duesberg 2003 op.cit.
9 Volberding PA, Lagakos SW, Koch MA, et al. Zidovudine in asymptomatic human immunodeficiency virus infection -- a controlled trial in persons with fewer than 500 CD4-positive cells per cubic millimeter. N Engl J Med 1990;322:941-9.
10 Lenderking WR, Volberding PA Evaluation of the quality of life associated with zidovudine treatment in asymprtomatic HIV infection NEJM 1994; 330:738-43
11 Aboulker JP. Preliminary analysis of the Concorde trial. Concorde Coordinating Committee. Lancet. 1993 Apr 3;341(8849):889-90
12 Ho D. Time to Hit HIV, Early and Hard. NEJM 333: 450-451, 1995
13 Kinloch-de Loes S, Hirschel BJ, Hoen B, et al. A controlled trial of zidovudine in primary human immunodeficiency virus infection. N Engl J Med 1995;333:408-13
14 Hoffman – Kamps HIV Medicine 2003 Flying Publisher pp 51-58
15 Mullis K. 'Dancing Naked in the Mind Field' (1998) First Vintage book edition.
http://www.garynull.com/Documents/Continuum/EruptiveTruthSpeakingGeneva.htm
16 Hellerstein M K, et al. Subpopulations of long-lived and short-lived T cells in advanced HIV-1 infection. J. Clin. Invest. 2003: 112:956–966.
Rosenberg YJ et al. HIV-induced decline in blood CD4/CD8 ratios: viral killing or altered lymphocyte trafficking? Immunol Today 1998; 19:10-7
17 Kinloch-de Loes S 1995 op.cit.
18 John Henkel FDA Consumer magazine July-August 1999 http://www.fda.gov/fdac/features/1999/499_aids.html
19 Hoffman 2003 op cit
20 Palella F J Jr, Delaney K M, Moorman A C, Loveless M O, Fuhrer J, Satten G A, Aschman D J and Holmberg S D 1998 Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV outpatient Study Investigators; N. Engl. J. Med. 338 853–860.
21 Hogg R S Montaner J S et al. Progression by Baseline CD4 Cell Count and Viral Load Rates of Disease After Initiating Triple-Drug Therapy. Jama 2001; 286 2568–2577.
22 COA. Aggiornamento Casi AIDS notificati in Italia. Dicembre 2004
23 Mocroft A et al. Changing patterns of mortality across Europe in patients infected with HIV” Lancet 1998; 352:1725-30.
24 Monini P, Ensoli B HIV protease inhibitors: antiretroviral agents with anti-inflammatory, anti-angiogenic and anti-tumour activity.J Antimicrob Chemother. 2003 Feb;51(2):207-11.
Tang AM et al. Improved antioxidant status among HIV-infected injecting drug users on potent antiretroviral therapy. JAIDS 2000 Apr 1;23(4):321-6.
25 Duesberg 2003 op.cit.
Atzori C In vitro activity of HIV P.I. against Pneumocystis c JID 2000;181:1629-34
26 Sackett D The sins of expertness and a proposal for redemption. BMJ 2000;320:1283.
27 V. Puro Lancet 2000; 355:1556-7.
28 Mocroft A et al. Decline in the AIDS and death rates…Lancet 2003;362:22-29
29 Mocroft A. et al. Causes of death in HIV infection: the key determinant to define the clinical response to anti HIV therapy. AIDS 200418:2333-7.
30 Mocroft a. et al. Aids across Europe, 1994-98: the EuroSIDSA study. Lancet 2000;356:291-6.
31 Reisler RB et al.Grade 4 events are important as AIDS events in the era of HAART. JAIDS 2003;34:379-86).
32 Yunzhen C, HO D et al. Virologic an immunologic chracterization of long-term survivors of HIV-1 infection. N Engl J Med 1995;332:201-8.)
33 Rode´s B. et al. Differences in disease progression in a cohort of long-term non-progressors after more than 16 years of HIV-1 infection AIDS 2004, 18:1109–1116
34 Drugs approved from the FDA: Tipranavir. June 2005 http://www.centerwatch.com/patient/drugs/dru880.html
35 Hoffman – Kamps 2003 op.cit.
36 Discordanza VL-CD4
• Ledergerber B et al. Clinical progression and virological failure on highly active antiretroviral therapy in HIV-1 patients: a prospective cohort study. Lancet 1999; 353:863–8.
• Nadine G. Pakker*, the INCAS Study Group. Immune restoration does not invariably occur following long-term HIV-1 suppression during antiretroviral therapy. AIDS 1999, 13:203–212.
• Lu W, Andrieu JM. HIV protease inhibitors restore impaired T-cell proliferative response in vivo and in vitro: a viral-suppression-independent mechanism. Blood. 2000 Jul 1;96(1):250-8. Comment in: Blood. 2001 Mar 15;97(6):1898-901.
• Aiko Okano et al. Discordant Movement of CD4-Positive T-Cell Count in HIV-1 Infected Patients with HAART Failure Jpn. J. Infect. Dis., 55, 62-65, 2002
• Grabar S et al. Clinical outcome of patients with HIV-1 infection according to immunologic and virologic response after 6 months of highly active antiretroviral therapy. Ann Int Med 2000. 133(6):401-410,)
• Sabin C et al. Discordant immunological and virological responses to HAART. Seventh Conference on Retroviruses and Opportunistic Infections, San Francisco, abstract 333, 2000.)
37 Deeks SG et al. Sustained CD4+ T cell response after virologic failure of protease inhibitor-based regimens in patients with human immunodeficience virus infection.J Inf Dis 181(3):946-953, 2000.
38 Fleming TR. Surrogate end points in clinical trias: are we being misled? Ann Int Med 1996;125:605-13.
39 Il n° dei CD4: parametro considerato nella definizione di AIDS (definizione dei CDC -1993) e valutato nei protocolli per l’inizio o la interruzione della terapia, per l’inizio o la sospensione della profilassi della toxoplasmosi e PCP.
40 immunoattivazione cronica
• Silvestri G, Feinberg MB. (2003) Op.cit..
41 Poli G, Fauci AS. The effect of cytokines and pharmacologic agents on chronic HIV infection. AIDS Res Hum Retroviruses. 1992 Feb;8(2):191-7.
42 Kremer H La rivoluzione silenziosa della medicina di cancro ed AIDS. Macroedizioni 2003.
Kidd P Th1/Th2 balance: the hypothesis, its limitation and implication for health and disease Altern Med Rev 2003;8:223-46
43 Passi S Aids :la nuova frontiera. Lombardo editore, 1996.
Eleni Papadopulos-Eleopulos. Reappraisal of AIDS - Is the Oxidation Induced by the Risk Factors the Primary Cause? Medical Hypotheses (1988) 25: 151-162 .
44 immunoattivazione = progressione
• Mette D. et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS 2003, 17:1881–1888.
Sousa AG. CD4 T cell depletion is linked directly to immune activation. J Immunol 2002;169:3400-6
Accelerated T cell turnover as a result of chronic immune activation:
• J. Clin. Invest. 112:821–824 (2003).
45 Long-term HIV suppression does not invariably lead to immune restoration.
• Nadine G. Pakker*, the INCAS Study Group. Immune restoration does not invariably occur following long-term HIV-1 suppression during antiretroviral therapy. AIDS 1999, 13:203–212.
46 Passi S Progressive increase of oxidative stress in advancing immunodeficiency. Continuum magazine 5(4):21-27.
47 HIV pathogenicity
• Poli G, Fauci AS. The effect of cytokines and pharmacologic agents on chronic HIV infection. AIDS Res Hum Retroviruses. 1992 Feb;8(2):191-7.
• Kalebic T, Kinter A, Poli G, Anderson ME, Meister A, Fauci AS. (1991). Suppression of human immunodeficieny virus expression in chronically infected monocytic cells by glutathione, glutathione ester, and N-acetylcysteine. Proc. Natl. Acad. Sci. U S A 88:986-990.
48 Montagnier L, Olivier R, Pasquier C, eds. Oxidative stress in cancer, AIDS and neurogenerative diseases. New York: Marcel Dekker Inc, 1998.
49 Passi S Ippolito F. op.cit. 1996,
50 Oxidative Stress, HIV and AIDS E. Papadopulos-Eleopulos et al . http://www.virusmyth.net/aids/data/epresimmun.htm
51 Kremer H. Op. Cit. 2003
52 S Kostensea et al. T cell expansions in lymph nodes and peripheral blood in HIV-1-infected individuals: effect of antiretroviral therapy AIDS 2001;15:1097-1107.
Tang AG et al. Improved antioxidant status among HIV infected on potent antiretroviral therapy JAIDS 2000;23:321-6
Monini et al. 2003 Op. cit.
Effetto proteasi
• Deeks SG et al. J Inf Dis 2000, op. cit..
• Lu W Blood. 2000 op. cit.
Inibizione virale da antiinfiammatori
• F. Pereira et al. Aspirin-like molecules that inhibit HIV 1 replication. Antiviral Research 2003. 58; 253–263.
Effetto verso i radicali liberi:
• GionchettiP ert al. Scavenger effect of sulfalazine, 5-amynosalycilic, and olsalazine on superoxide radical gneration. Dig. Dis. Sci 1991;36:174-8
53 Lu W. Blood 2000 op. cit.
54 effetto immunomodulazione HAART
• Andre P et al. An inhibitor of HIV-1 protease modulates proteasome activity, antigen presentation, and T cell responses. Proc Natl Acad Sci U S A. 1998 Oct 27;95(22):13120-4.
• Atlas A et al. Effects of potent antiretroviral therapy on the immune activation marker soluble CD27 in patients infected with HIV-1 subtypes A-D. J Med Virol. 2004 Mar;72(3):345-51.
55 Cortisonici
• J-M Andrieu & W Lu. Long-term [10 years!] clinical, immunologic and virologic impact of glucocorticoids on the chronic phase of HIV infection BMC Medicine 2004, 2:17
56 Proprietà dell’aspirina
• EN Sibanda et al. AIDS Vaccines and Related Topics, 2004 (in press).
Aspirina ed AIDS (cd4<200 cells).
• GA Stankzuk, et al. Acetyl salicylic acid (aspirin), micronutrients and chloroquine in the management of the Acquired Immunodeficiency Syndrome (AIDS). Cent Afr J Med 2002;48(3/4):42-9.
EN Sibanda et al. Acetyl salicylic acid (ASPIRIN) increases the CD4+ T lymphocytes and suppresses TNF-a in HIV-1 infected patients: Results of a 12 month, three-arm, placebo controlled pilot study. AIDS Vaccines and Related Topics, 2004 (in press).
57 salazopirina
• Disla E, Rhim HR, et al.. Improvement in CD4 lymphocyte count in HIV-Reiter's syndrome after treatment with sulfasalazine. Rheumatol 1994 Apr;21(4):662-4
58 P. Duesberg: http://www.virusmyth.net/aids/index/pduesberg.htm E. Eleopulos Papadopulos: http://www.virusmyth.net/aids/index/epapadopoulos.htm



