from Leadership Medica n.2/2001
ABSTRACT
Pier Mario Biava, Daniele Bonsignorio, Mirjam Hoxha, Alberto Frosi, Monica Impagliazzo
Five tumor cell lines of different origin (glioblastoma, melanoma, kidney adenocarcinoma, breast carcinoma and lymphoblastic leukemia) were treated in vitro with the extracts from zebrafish embryos collected at four different developmental stages. All cell lines responded with a significant slowing down of the proliferation when treated with the extracts taken during the stages of cell differentiation, while no slowing effect was observed when they were treated with the extract taken from a merely multiplicative stage. These results suggest that a complex network of molecular factors during embryo differentiation may help abnormally proliferating cells to normalize their cycle, and that the administration of embryonic cell differentiation factors may be a useful tool in cancer therapy. On the other hand, it is known that the stem cells can be differentiated into different types of cells in relationship to different kinds of embryonic microenvironment. Since this network of cell differentiation factors may normalize the altered expression of genes, we suggest it as a physiological gene therapy.
Introduction
 The evidence obtained from studying the interactions between embryonic tissues and tumor cells suggests that tumor growth is reduced or suppressed when tumors are treated with embryonic extracts taken during organogenesis . In fact, it was demonstrated that the growth of the primary tumor and the formation of pulmonary metastases in C57BL/6 syngeneic mice inoculated with 1x 106 Lewis lung carcinoma cells were strongly delayed when the mice were treated with pregnant uteri as well with embryonic extracts at the early stages of organogenesis (1,2,3). More precisely, it was demonstrated that the abnormal growth of cell clones during embryo organogenesis in mammals is prevented by low–molecular weight substances present in the pregnant uterus microenvironment. Previous experiments in our laboratory showed that the pregnant mouse uterus extracts are able to inhibit the growth of Lewis lung carcinoma cells in vivo (1). In addition, more recent experiments in vitro showed that the pregnant pig and mouse uterus extracts slow down the proliferation rate of several established human tumor cell lines (4). Therefore, the interaction between mother and embryo seems to be important for normal development and for preventing pathological cell growth. Embryo itself seems to prevent the abnormal multiplication of tumor cells. The fact that embryonic development and tumorigenesis are closely correlated is now well accepted: they both share several pathways and regulatory molecules, so that cancer can be viewed as a developmental deviation susceptible to control by the regulators of cell differentiation (5,6). The main effect of the in vitro treatment of tumor cell lines with the extracts from oviparous embryos is the activation of p53 expression, as observed by the immunohistochemical and flow cytometry techniques after the treatment of glioblastoma, melanoma and hepatocarcinoma cell lines with the extracts from fish embryos (7). In order to assess whether these embryonic extracts affect tumor cell proliferation, the present work aims to determine the in vitro effects of zebrafish embryo extracts at four distinct developmental stages on the growth of five tumor cell lines.
The evidence obtained from studying the interactions between embryonic tissues and tumor cells suggests that tumor growth is reduced or suppressed when tumors are treated with embryonic extracts taken during organogenesis . In fact, it was demonstrated that the growth of the primary tumor and the formation of pulmonary metastases in C57BL/6 syngeneic mice inoculated with 1x 106 Lewis lung carcinoma cells were strongly delayed when the mice were treated with pregnant uteri as well with embryonic extracts at the early stages of organogenesis (1,2,3). More precisely, it was demonstrated that the abnormal growth of cell clones during embryo organogenesis in mammals is prevented by low–molecular weight substances present in the pregnant uterus microenvironment. Previous experiments in our laboratory showed that the pregnant mouse uterus extracts are able to inhibit the growth of Lewis lung carcinoma cells in vivo (1). In addition, more recent experiments in vitro showed that the pregnant pig and mouse uterus extracts slow down the proliferation rate of several established human tumor cell lines (4). Therefore, the interaction between mother and embryo seems to be important for normal development and for preventing pathological cell growth. Embryo itself seems to prevent the abnormal multiplication of tumor cells. The fact that embryonic development and tumorigenesis are closely correlated is now well accepted: they both share several pathways and regulatory molecules, so that cancer can be viewed as a developmental deviation susceptible to control by the regulators of cell differentiation (5,6). The main effect of the in vitro treatment of tumor cell lines with the extracts from oviparous embryos is the activation of p53 expression, as observed by the immunohistochemical and flow cytometry techniques after the treatment of glioblastoma, melanoma and hepatocarcinoma cell lines with the extracts from fish embryos (7). In order to assess whether these embryonic extracts affect tumor cell proliferation, the present work aims to determine the in vitro effects of zebrafish embryo extracts at four distinct developmental stages on the growth of five tumor cell lines.
Materials and Methods
Zebrafish embryo extracts
Embryos of zebrafish at the following developmental stages:
- nearly 1000 blastomeres;
- 50% epiboly;
- 5 somites and
- 20 somites were collected and dissolved with a turboemulsionator in cold PBS.
The extracts, named respectively “ stage 1k”, “stage I”, “stage II” and “stage III”, were prepared as 1 mml stocks in a glyceralcoholic solution composed as follows: 85 % glycerol and 15 % absolute ethanol, and conserved at 4°C until use.
Culture and treatment of tumor cell lines with the crude zebrafish embryo extracts
Human tumor cell lines A172 (glioblastoma), A375 (melanoma), ACHN (kidney adenocarcinoma), ZR75.1 (breast carcinoma) and H9 (lymphoblastic leukemia) were obtained from the Istituto Zooprofilattico Sperimentale in Brescia, Italy, and expanded in our lab. All cells were grown in culture media supplemented with 10% Fetal Calf Serum, 1 mM Na-piruvate, 2 mM L-Glutamine and antibiotics Penicillin / Streptomicin. Culture media Eagle’s MEM, Dulbecco’s MEM and RPMI1640, Na-piruvate, L-Glutamine, antibiotics and PBS were purchased from Labtek Eurobio; Fetal Calf Sera were purchased from Celbio. In order to establish which dilutions of the glycerol and alcohol solvents were without effects, preliminar cell proliferation assays were performed after the treatment of the cell lines with several dilutions of pure glyceralcoholic solution (data not shown). We observed that the rate of cell proliferation in a 1: 50 dilution of glyceralcoholic solution was not significantly different from that of cells growing in a normal medium. All cell lines were seeded in 60 mm Ø petri dishes, except H9 cells, which were seeded in 25 mm flasks. After seeding, all cells were allowed to grow for 24 hours before treatment. Treatments were performed by adding the crude zebrafish embryo extracts at a 1:50 dilution in fresh medium to cells. The total amount of protein per treatment was 100 ng. Cell counts were performed manually with the Trypan Blue method at 0, 24 and 48 hours after the treatment. For each experiment, ten counts per treatment were made.
Risults
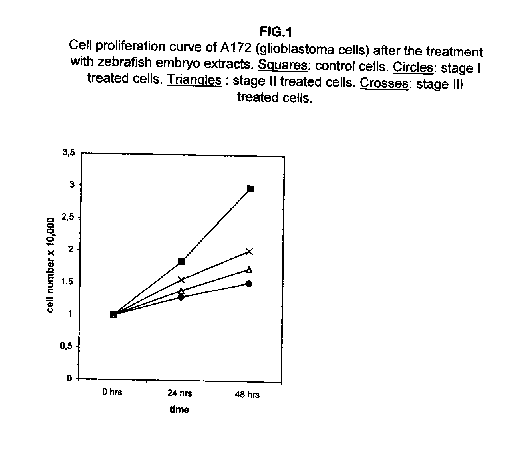
Figure 1 shows that the cell proliferation curves were slowed down remarkably in glioblastoma A172 cells after the treatment with the I, II and III stages of zebrafish embryo extracts, the stage I being the most effective. A consistent slowing effect is already visible 24 hours after the treatment with all three extracts, augmenting at 48 hours.
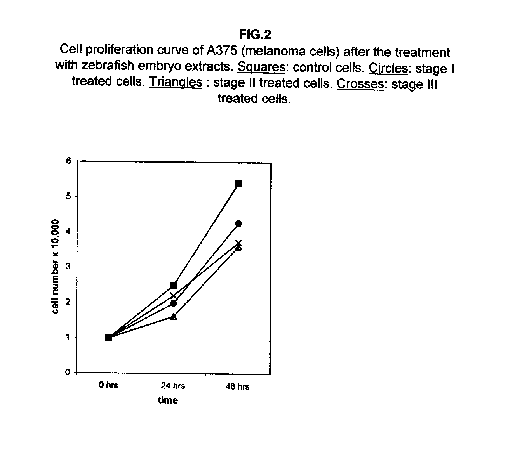
In melanoma A375 cells, the proliferation at 24 hours after the treatment is less affected, but it also shows a remarkable slowing down at 48 hours (figure 2).
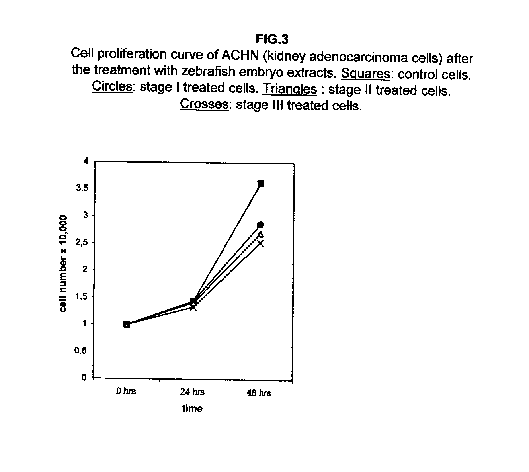
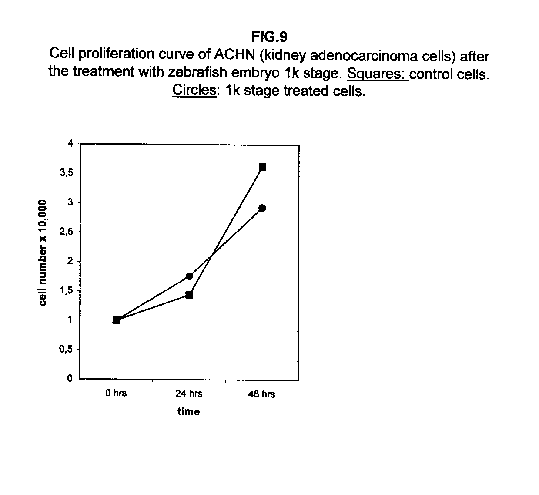
Stages II and III extracts are the most effective. In treated kidney adenocarcinoma ACHN cells, no effect on the growth is observed 24 hours after treatment with respect to control cells, but all three stages result in a slower cell proliferation 48 hours after the treatment (figure 3).
In breast carcinoma ZR.75.1 cells, stage I extract almost blocks cell growth, while proliferation curves of stages II and III treated cells after 24 hours even fall, suggesting that cell killing rather than a growth slowing mechanism may occur (figure 4).
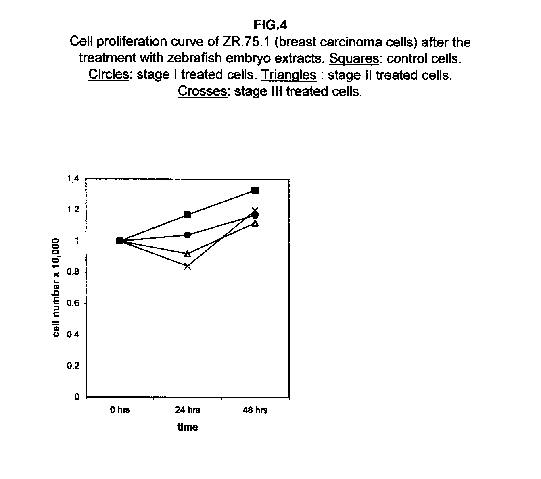
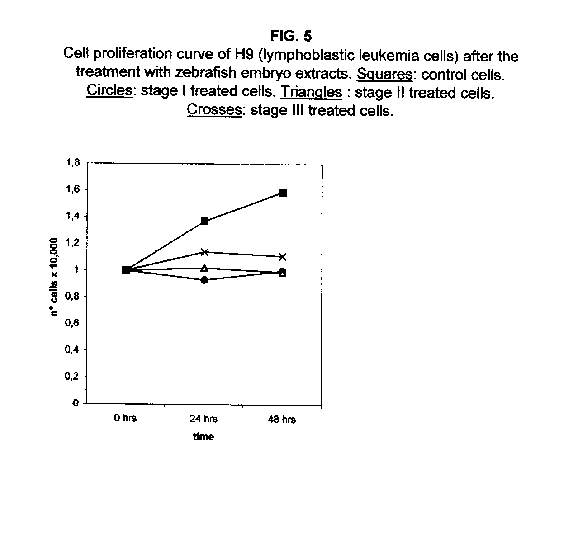
Forty-eight hours after the treatment, however, the stage II and III proliferation values rise, approaching that of the stage I treated cells. The proliferation rates of H9 cells treated with all three stages were almost blocked 48 hours after the treatment (figure 5).
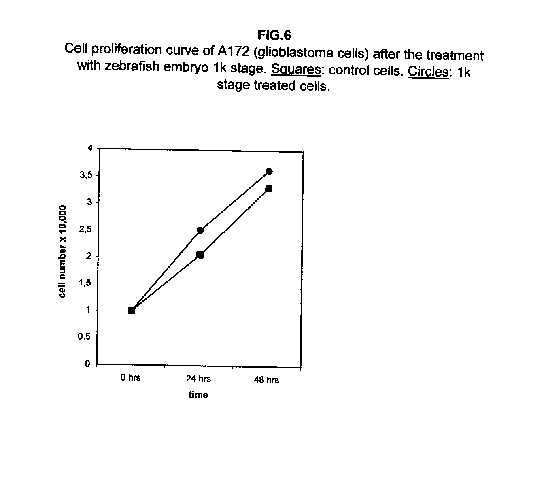
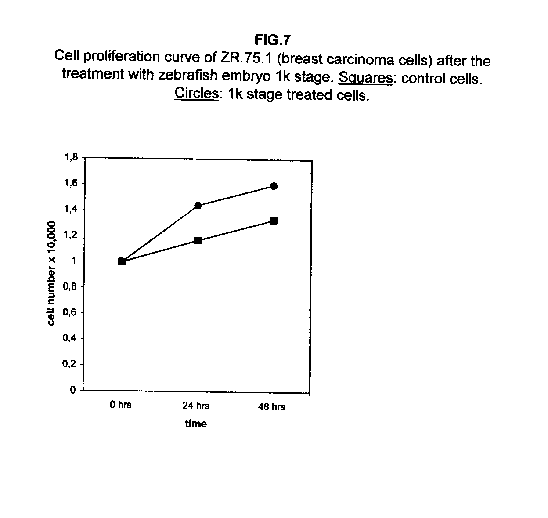
The treatment of all cell lines with an expected proliferating stage, namely, the 1k stage, showed that in all cases the proliferation was the same as the control cells, if not higher. In A172 and ZR.75.1 cells, the proliferation rate of 1k stage treated cells was faster both 24 and 48 hours after treatment (figures 6 and 7).
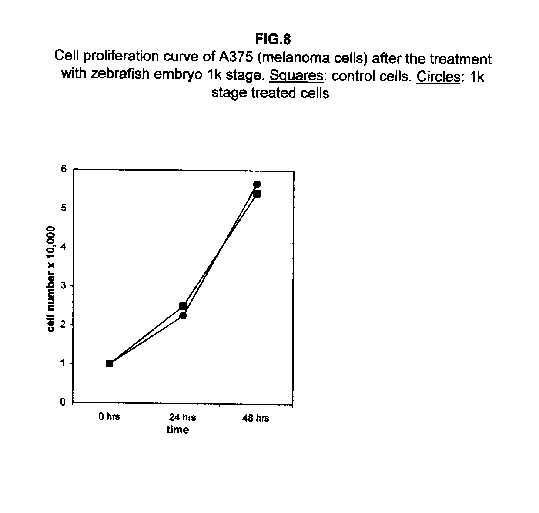
In A375 cells, the two curves nearly collided (figure 8), while ACHN cells showed an intermediate behaviour, the 1k stage treated cells proliferation value being higher than control cells at 24 hours and lower at 48 hours (figure 9).
Discussion
Our results demonstrate that molecules present during some decisive stages of embryonic differentiation are able to delay the growth of different tumor cell lines in vitro. Forty-eight hours after the treatment, all cell lines analysed showed a significant decrease of the proliferation curve. The lymphoblastic leukemia (H9) cell line showed the strongest response to all three differentiation stages (I, II, III), since the proliferation was nearly stopped. The slowing efficacy of the extracts varied with the cell line analyzed: apart from H9, the most responsive cells were the glioblastoma A172 and the breast carcinoma ZR.75.1 lines, which already showed a remarkable response already 24 hours after the treatment. The melanoma A375 line showed a slightly weaker response, while the treatment was effective on the proliferation rate of kidney adenocarcinoma ACHN cells only after 48 hours. This evidence may suggest that not all kinds of tumor respond in the same way to treatment with embryonic extracts. In any event, the slowing percentage values taken 48 hours after the treatment were never less than 25 %. While stage III (24 hours of development) had a somewhat uniform effect on all tumor cells (the slowing percentage ranging between 49 % in A172 and 39 % in ZR.75.1 and A375), the slowing effects of stages I (50 % epiboly developmental stage, corresponding to the gastrulation start) and II (5 somites developmental stage) were more fluctuating even though more marked: stage I slowing percentage peaks 74% in A172, while stage II slowing percentage reaches 67 % in ZR.75.1 and 63% in A172. On the other hand, molecules extracted from a merely multiplicative stage , such as the 1k stage (segmentation stage), do not have any effect on the proliferation of the same cells. Thus, cell differentiation is a key process in understanding the behaviour of both normal and tumor cells. The tumor cell genome is normally affected by a dramatically high number of altered genes, most of which playing an important role in normal embryo development. In fact, during tumorigenesis certain embryonic genes are activated (proto-oncogenes) or mutated (oncogenes), leading the cell to an uncontrolled multiplication program. Probably in tumor cells all or part of the program of cell differentiation has been disactivated. The erasure of this program leads to a renewed expression of embryonic genes normally active in the multiplication program. Thus, tumor cells can be considered as cells sharing gene configurations similar to those of cells of developing embryos. Actually, it is known that tumor and embryonic cells share some surface antigens. Just recently a novel carbohydrate antigen has been discovered, shared by several tumor cell lines and Xenopus laevis embryo (8,9). According to this point of view, box 1 describes a model of cell differentiation which predicts the number of types of the completely differentiated cells and of different kinds of tumors. According to this model, if we remember that embryonic cells are multiplying between two stages of differentiation, we can consider tumor cells as embryonic mutated cells in which the programs of cell multiplication and cell differentiation are no longer correctly coupled. Thus, they behave as a computer in loop, repeating only the multiplication program. More precisely tumor cells can be considered, in according to the model of the box 1, as mutated stem cells (9 types in the reported model), or as mutated committed stem cells (27 types), or as mutated differentiating cells (81 types), in relationship with their degree of malignancy. The factors present in the differentiating tissues of embryos are able to restore the original conditions, normalizing and differentiating the undifferentiated mutated cells. This normalization may be in charge directly of key-role regulators and genes of cell cycle and differentiation. These regulators are probably organized in a network or constitute a family of similar substances cooperating in complex cascades. Thus, the multiplication of tumor cells may be controlled only when this network is complete. In fact, according to a complex model, one or few molecules contribute only in a modest way in giving complete information and in regulating the complex gene alterations in tumor cells. The sum of all the factors surrounding the cell is the microenvironment, which is decisive in regulating the multiplication and the differentiation of both normal and tumor cells. An embryo holds the most effective microenvironment, being able to lead the multipotent stem cells to a complete differentiation (10,11,12). On the other hand, it is known that embryonic microenvironment can differentiate the stem cells in different ways. In fact, it was demonstrated that the stem cells can be committed into different types of cells in relationship to different kinds of factors: for example, neural stem cells can be differentiated into cells of the hematopoietic lineage when put in contact with the hematopoietic microenvironment (13), or into skeletal muscle cells when put in contact with factors of skeletal muscle differentiation (14). The same results are obtained with liver stem cells (15). Previous work (7) has demonstrated that embryonic extracts taken from cell differentiation stages are able to activate the expression of the p53 tumor suppressor gene in different tumor cell lines. The activation of p53 may be functionally linked to the slowing down of the proliferation rate of tumor cells, as observed in this work, although we cannot exclude that other components of the cell cycle are involved . At present we are conducting experiments to elucidate the role of pRB in this regulation. The pRb seems to be regulated by the embryonic extracts: so the slowing down of tumor cells proliferation curves can be in charge of a multi-genes regulation (yet unpublished data). We are now studying also the role of telomerase on the observed slow down of tumor cells proliferation curves after the treatment with embryonic extracts. In fact it is known that differentiated cells contain a lower telomerase levels than stem cells. So it is probable that the treatment with embryonic extracts can reduce the level of telomerase in tumor cells. We know from literature that the creation of human tumor cells with defined genetic elements involves exactly p53, pRb and telomerase (16). So the treatment with the regulators of cells differentiation is not only able to differentiate the normal stem cells, but probably also the mutated stem cells, the mutated committed stem cells and the mutated differentiating cells, by passing over the mutations, that give rise to malignancy. In any event, the treatment of cancer patients with embryonic extracts taken during specific stages of cell differentiation, not of cell multiplication, could represent a promising new therapeutic tool. It could be also considered as a harmless, inexpensive physiological gene therapy, without the various drawbacks of conventional gene therapy.
(traduzione dell'autore)
Pier Mario Biava
Fondazione per la Ricerca delle Terapie Biologiche del Cancro.
Ospedale Civile Sesto San Giovanni - Milano
Bibliography
1) Biava PM, Fiorito A, Negro C, Mariani M: Cancer Letter 41, 265-270 (1988)
2) Biava PM, Carluccio A : La Medicina Biologica 2-4 (1994)
3) Biava PM, Carluccio A : Biol Medizin 5: 247-249 (1995)
4) Biava PM, Bonsignorio D, Hoxha M : J Tumor Marker Oncol: 15:223-233 (2000)
5) Zhang P : Curr Opin Cell Biol 11: 655–662 (1999)
6) Potter CJ, Turenchalk GS, Xu T : Trends Genet 16: 33-39 (2000)
7) Biava PM, Carluccio A : J Tumor Marker Oncol 12: 9-15 (1997)
8) Klavins JV, Sell S, Fuchs A : J Tumor Marker Oncol 11: 36 (1996)
9) Zhang S, Sell S, Livingston PO, Klavins JV : J Tumor Marker Oncol 12: 52 (1997)
10) Stewart TA, Mintz B: Proc Natl Acad Sci USA 78: 6314-6318 (1981)
11) Feijen A, Goumans MJ, Van den Eijnden-van Raaij AJ: Development 120: 3621-3637 (1994).
12) Andrews PW, Damjanov I, Berends J, Kumpf S, Zappavigna V, Mavilio F, Sampath K : Lab Invest 71(2): 243-251 (1994).
13) Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL : Science 283 (5401): 534–537 (1999).
14) Galli R, Borello U, Gritti A, Minasi MG, Bjornson C, Coletta M, Mora M, De Angelis MG, Fiocco R, Cossu G, Vescovi AL: Nat Neurosci 3(10): 986-991 (2000).
15) Sell S : Cellular origin of liver stem cells. 16th Int. Conf. on Human Tumor Markers, Budapest, June 13-16 (1999).
16) Hahn W.C. Nature Vol. 400 (1999)



