da Leadership Medica n. 254 (2007)
Abstract
Cellular and molecular magnetic resonance imaging (MRI) is experiencing huge developments thanks to high spatial and contrast resolution of the technique, but also thanks to the many research studies concerning new contrast media developed in recent years. Even today, with the contrast agents in use, it is possible to label some cells and trace their tracks in living organisms. However, it goes without saying that the most exciting frontier in this field is to develop contrast agents which selectively detect specific molecules typical of a certain tissue. This manuscript is made up of an introductive part followed by two examples of cellular MRI that are possibile nowadays and represent important research fields at our Institution. In the conclusive part of the manuscript, attention will be focused on magnetic resonance spectroscopy (MRS), which represents a molecular imaging technique. In particular, it will be illustrated how cardiac energetic metabolism may be studied by means of 31P MR Spectroscopy, one of the main extracranic applications of this imaging technique.

Introduction
Magnetic Resonance Imaging (MRI) is gaining importance in the field of cellular and molecular imaging.
The outstanding research developments of contrast media, the use of high intensity magnetic fields and of micro-coils that allow very high spatial resolution, the growing awareness of the enormous possibilities of MR
Spectroscopy, are giving MRI the possibility to study in vivo cellular and sub-cellular events. It is mandatory to gradually pass from anatomical imaging to cellular or even molecular imaging in the era of stem cells for tissue damage healing, gene and cellular theraphies for present and future applications in oncology.
For these reasons, it is very important to have availability of a reliable imaging technique that can be exploited, for example, in the field of tissue regenerating medicine with stem cells, to follow migration and homing of in vivo injected cells, to test in vivo efficacy of different cellular subtypes and different therapeuthic strategies. In this setting, MRI has a strategic role thanks to its intrinsic charachteristics: in fact, it allows non-invasive 3D imaging, with high spatial and contrast resolution, of small amounts of cells and offers the advantage of easy and reproducible imaging experiments in animals as well as in humans.
Cellular Mr imaging and tumor immune-therapy
An emerging application field of cellular MRI is tumor immune-therapy using dendritic cells (DCs) based vaccines. In order to realize optimal DCs therapies it is fundamental to obtain reliable data concerning in vivo cellular homing. In our Institution (Radiology Department in collaboration with Cancer Immune-Therapy Research Unit: Prof. A. Manfredi) we develop DCs labelling with superparmagnetic iron oxide nanoparticles (SPIO), in order to obtain the direct depiction of traffic of intravenously injected DCs in vivo, in mice. We used a clinically approved SPIO contrast agent (Endorem®, Guerbet) to label murine C57BL/6 bone marrow derived DCs. DCs were incubated with SPIO for 16 hours, in order to obtain an adequate contrast uptake. Different samples of 2 millions of DCs were incubated with growing SPIO concentrations (0, 80, 160, 320, 640 g/ml) and underwent in vitro MRI, to test the smallest contrast agent concentration needed to obtain proper labelling. Respective signal to noise ratios (S/N) were measured: based on these data the best SPIO concentration to label DCs for in vivo experiments was chosen. Successively, SPIO labelled DCs were intravenously injected into 4 syngeneic lymphoma-bearing mice. All mice underwent in vivo MRI, during general anaesthesia. MR imaging has been repeated four times in each mouse, before and 1, 24, 48 hours after intravenously injection. The S/N of liver, spleen and tumor was measured. Tissue homing of DCs was verified by pathology analysis, that represents the gold standard technique. Progressive decrease of S/N was observed moving from the samples of DCs incubated with 0mg/ml to those incubated with 320mg/ml of SPIO. No difference was found between 320 and 640mg/ml. (you see figure). SPIO concentrations used weren't toxic for DCs and did not suppress their ability to present exogenous proteins like OVA to T cells and to activate them. Turning to the results of in vivo experiments, significant decrease of liver and spleen S/N was observed after SPIO-DCs i.v. injection.
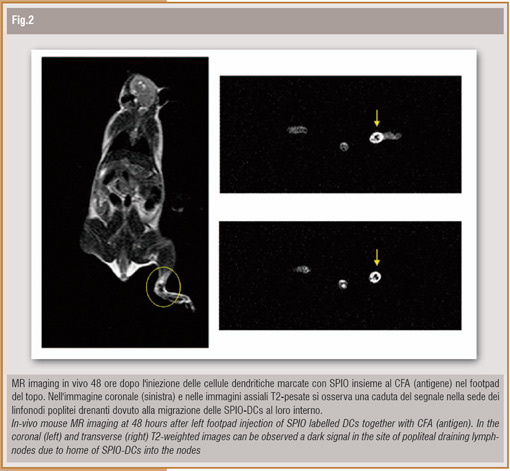
The S/N of tumor wasn't different before and after i.v. injection. Histological examinations confirmed the presence of the injected SPIO-DCs in liver and spleen and their absence in the tumor, according to MR imaging results. These results suggest that DCs can be labelled with SPIO, that is internalized without toxicity and specific cellular abilities impairment; moreover, it is possible to perform in vivo tracing of SPIO labelled DCs by MRI. DCs homing in host nodes is fundamental in order to maximize the interaction between DCs and antigen specific T-cells, that is of pivotal importance for tumor immune-therapy success. For this reason, in a subsequent experiment, we evaluated the possibility to trace DCs in vivo migration in the nodes. After mouse footpad subcutaneous SPIO-DCs and antigen injection we observed a clear signal loss in the site of the draining nodes (Figure); histological nodes analysis confirmed SPIO-DCs presence. An experimental study similar to ours have been recently published by Baumjohann D. et al (1) which demonstrate that it is possible to non invasively assess DCs migration in the draining nodes by means of MRI, using a clinically approved contrast agent.
MR cellular imaging and pancreatic islet transplantation
A promising alternative to insulin administration it is to cure T1DM by means of pancreatic islet transplantation. This treatment, available only for a very small minority of patients due to the very limited availability of human tissue from donors, restores the endogenous insulin secretion achieving insulin independence in more than 80% of cases. Moreover, a better control of glycated HbA1c, a reduced rate of recurrent hypoglycemia and a reduction of diabetic complications are additional advantages observed in pancreatic islet recipients, regardless of insulin independence. One major problem is however that the long-term success of the graft is not always guaranteed. Very little is known about the destiny of the islets in human liver after their infusion into the portal vein. It is therefore of critical importance to develop a reliable non-invasive islets imaging modality for the follow-up of the graft fate and for studying the relationships between transplanted islet mass and insulin independence, graft function and diabetes complications. Such an imaging tool would also allow to study in-vivo the effect of different immunomodulatory strategies on islet mass preservation or reduction due to rejection. Recently, several centers investigated the possibility to label the islets with superparamagnetic iron oxide nanoparticles (SPIO) and to image them using MRI with very promising results. In our institution (Radiology department in collaboration with Islet Research Unit: Dr. E. Bonifacio/Dr. M.L. Malosio) we labeled human and murine islets using clinically approved SPIO particles (Endorem®, Guerbet). The vitality and the ability to secrete insulin, assessed in vitro, did not change after labeling. We performed both in-vitro and in-vivo MRI studies using a 1.5 T scanner. Our in-vitro imaging results showed a good correlation between signal loss and increasing numbers of islets. In our initial in-vivo studies, we were able to detect labeled islets transplanted under the kidney capsule of mice made diabetic by means of streptozotocin, which had become normoglycemic after transplantation. The future objectives of our research are to better define the behavior of SPIO labeled islets transplanted in diabetes animal models and to transfer the developed MRI technique to humans.
Mr spectroscopy and molecules measurements
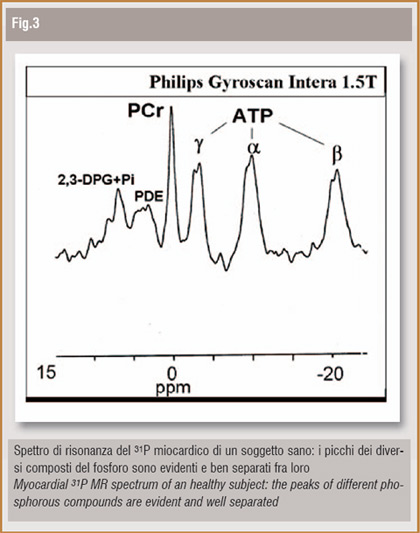
As already mentioned, the intrinsic charachteristics of MR allow realistic possibilities of molecular imaging. The most important example is MR-Spectroscopy (MRS). MRS is based on nuclear magnetic resonance phenomenon, as well as MRI, and thus represents a non-invasive and safe technique for in vivo human and animal studies. Every nucleus with a magnetic moment other than zero, if positioned in a static magnetic field and excited by a proper radiofrequency impulse, produces a signal with a typical wavelenght; if the nucleus belongs to a molecule, its resonance signal will be slightly different, depending on the molecular structure itself. This phenomenon, known as "chemical shift ", allows quantitative and qualitative analysis of molecular tissue composition. The most interesting nuclei, from a clinical point of view, are phosphorous (31P), hydrogen (1H) and carbonium (13C). Nowadays the most innovative application in body imaging is 31P MR- Spectroscopy (31P-MRS). (you see figure). 31P is a fundamental component of molecules implicated in energetic cardiac metabolism and the Phosphocreatine (PCr)/ATP ratio is considered to be the most reliable and reproducible parameter to measure cardiac metabolism. In our Institution professional athletes, hypertrophic cardiomyopathy (HCM) patients, type-1 diabetic and uremic (T1MD-Ur) patients (3) and chronic heart failure (CHF) patients (4) have been studied on a 1.5 T magnet (Philips). Our results showed normal cardiac energetic metabolism in benign myocardial hypertrophy in athletes, regardless different training habits; on the contrary, metabolism was altered in HCM patients. In T1DM-Ur subjects, cardiac metabolic impairement anticipates functional heart alterations and proper diabetes and uremia theraphy with kidney-pancreas transplantation allows to restore a normal cardiac metabolism. In CHF patients PCr/ATP ratio is altered in regards to symptoms and functional heart status. We can conclude that, according to our experience as well as literature studies, spectroscopy imaging adds to a conventional cardiac MR exam some exclusive informations, which seem to be very useful in early diagnosis, theraphy monitoring and understanding of pathophisiological basis of important myocardial diseases.
Prof. Alessandro del Maschio
Full Professor and Chairman of Radiology
Università Vita-Salute S. Raffaele - Milano, Italy
Bibliografia
1. Baumjohann D, Hess A, Budinsky L, Brune K, Schuler G, Lutz MB.
In vivo magnetic resonance imaging of dendritic cell migration into the draining lymph nodes of mice. Eur J Immunol. 2006 Sep;36(9):2544-55.
2. Evgenov NV, Medarova Z, Dai G, Bonner-Weir S, Moore A.
In vivo imaging of islet transplantation. Nat Med 2006 Jan;12(1):144-8.
3. Perseghin G, Fiorina P, De Cobelli F, Scifo P, Esposito A, Canu T, Danna M, Gremizzi C, Secchi A, Luzi L, Del Maschio A. Cross-sectional assessment of the effect of kidney and kidney-pancreas transplantation on resting left ventricular energy metabolism in type 1 diabetic-uremic patients: a phosphorous-31 magnetic resonance spectroscopy study. J Am Coll Cardiol. 2005 Sep 20;46(6):1085-92.
4. Fragasso G, Perseghin G, De Cobelli F, Esposito A, Palloshi A, Lattuada G, Scifo P, Calori G, Del Maschio A, Margonato A. Effects of metabolic modulation by trimetazidine on left ventricular function and phosphocreatine/adenosine triphosphate ratio in patients with heart failure. Eur Heart J. 2006 Apr;27(8):942-8. Epub 2006 Mar 1.



