from Leadership Medica n. 7/2007
Abstract
The surgical indication for abdominal aortic aneurysms (AAA) depends on several factors of local and, most important, general relevance, however there is a general consent on the necessity of operating the asymptomatic AAAs with diameter > 5 cm. The traditional intervention consists of performing, under general anesthesia and through a xifo-pubic median laparotomic access, the endoaneurysmectomy and placing a prosthesis normally dacron knitted. It is considered an high level surgery with morbidity and mortality around 3% and a post-operative course quite serious. To improve results and attain a less demanding course, have been employed aortic endoprostheses which are introduced by an inguinal access under epidural anesthesia, or has been used a third approach that is the mini-invasive aortic surgery that can treat AAAs through a mini-laparotomy and video-laparoscopy.
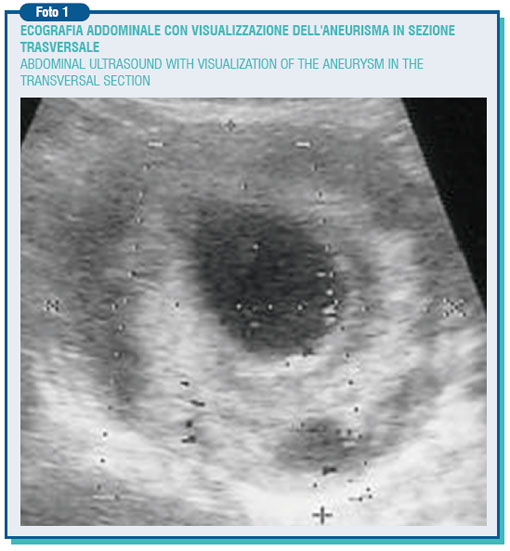
Aneurysm is the permanent and focal artery expansion with diameter increase of at least 50% compared with its normal value. Such expansion is due to a change in the artery structure, in which becomes apparent a partial disruption of its elastic and muscular components. The expansion leads to the formation of turbulences together with the slow-down in the blood flow, resulting in a wall thrombosis. The aneurysm pathogenesis is likely to have several causes yet the atherosclerotic disease represents the most important one. More than 90% of the abdominal aneurysms (AAA) show aortic indeed atherosclerotic features.
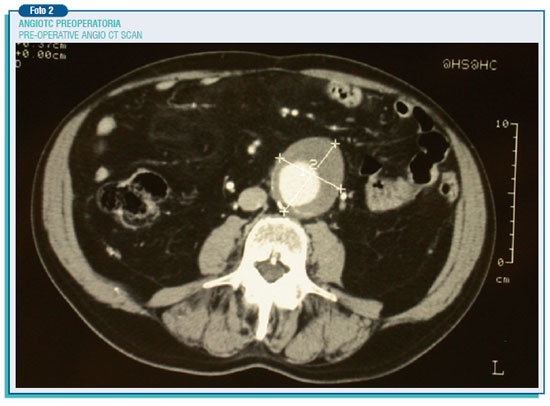
The most frequent AAAs' site is located below the origin of renal arteries, then they go generally upwards to the aortic division. Their frequency, especially in males, keeps on rising due to a widespread prevalence of the atherosclerotic disease, the increase of elderly people and also the development of non-invasive diagnostic techniques such as ultrasound which are expected to become screening tests for the over-65-year population.
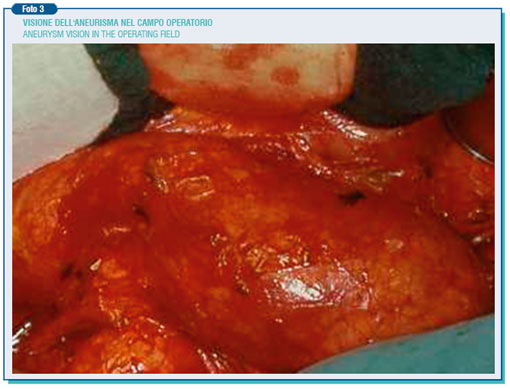
The abdominal aorta below the kidney has a cross-diameter of around 2 cm, however this varies with age, weight and body surface area. A diameter greater than that is called ectasia (i.d. expansion), whereas only when it goes beyond 3,5-4 cm it is called aneurysm. Generally the aneurysm is asymptomatic but, as the result of steady expansion (0.3-0.4 cm/year increase), it evolves towards the break-up, which sometimes occurs suddenly.
This risk increases with the rise of the AAA diameter: 4.1% under 5 cm, 6.6% between 5 and 7 cm, and 19% over 7 cm. The broken aneurysm has got a mortality that tops even to 94%, when also the deaths happened before the hospitalization are accounted for. In any case it is an utterly appalling event often involving, in the survivors, a very long period of recovery and the reduction in the quality of life as a consequence of various and different complications. Therefore, there is a widespread consent on the need to operate the asymptomatic aneurysms having a diameter more than 5 cm also because the post-operative mortality is about 3%, whereas there is no agreement about surgical indications for the so-called small aneurysms (lower than 5 cm).
The surgical indication certainly relies on several factors either of local relevance, such as a rapid growth (more than 0.5 cm per year), blisters occurrence (localized enlargements easily breaking), or most important of general relevance such as heart and lung failure, renal failure, expectancy of life lower than 2 years, and the association with coronary or carotid lesions. The first surgical intervention was carried out by Dubost on 23.03.1951, although the currently used technique was introduced by Creech in 1966, who did not perform the aneurysm resection as a tumor, but an endoneurysmectomy with alloplastic graft insertion.

Through a median xifo-pubic laparotomy under general anesthesia, the anterior and left side of the aneurysm is isolated, then going upward to retrieve and separate the aneurysm neck in the aorta below the renal arteries’, where its diameter is nearly normal. Particular attention must be paid to the skeletrization of the preaortic nervous plessus during the isolation of the aortic bifurcation and, most important, of the left common iliac artery, as the injury of this plessus can lead to sexual dysfunction characterized by retrograde ejaculation.
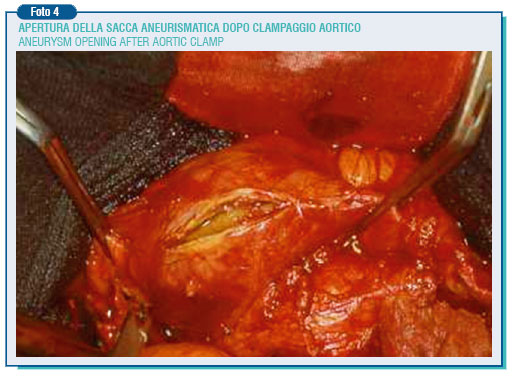
Downward to the aneurysm based on its extension, the common iliac arteries or both the external and internal iliac artery have to be isolated. The inferior mesenteric artery is isolated on the aneurysm left side. If it is pervious, it has to be assessed the presence or not of a Doppler ultrasound signal coming from the sigmoid colon after clamping: the disappearance of the signal makes compulsory to reimplant the mesenteric artery into the prosthesis to avoid ischemic intestinal injuries. After treatment with heparin, the aneurysm is clamped at its extremities and the incision is made along its left side, the clots and necrotic content are removed and the bleeding lumbar and sacral arteries' openings are sutured from the inside.
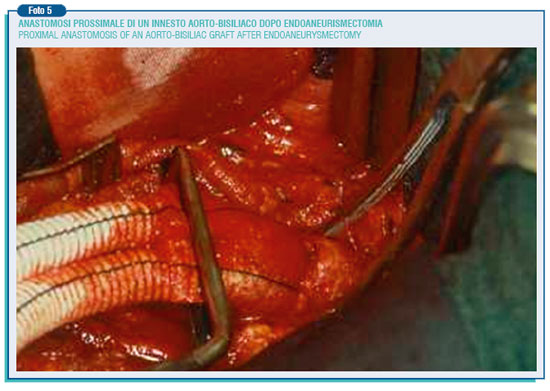
Then it has to be chosen the prosthesis, straight or bifurcated, slightly smaller than the aortic neck in order to prevent the possibility of future enlargement. These are prostheses of 16-18 mm diameter, woven or knitted in Dacron woven or knitted, and sealed with albumin, collagen or gelatine so to avoid pre-coagulation.
The proximal and distal anastomosis are performed in a end-to-end way. Finally the aneurysm wall is hand-sewn around the prosthesis to prevent the straight contact between the prosthesis and the duodenum, and thus the possible formation of an aortic-enteric fistula. As said before, the operating mortality is around 3%, but the above described intervention entail a certain degree of danger, especially in patients defined for their general conditions at "high surgical risk".
The wide surgical incision, going along the whole abdomen, the presence of the nose-gastric catheter for 2-3 days until bowel movements are restored, and the bladder catheter make the post-operative course rather painful and demanding. Moreover the patient must stay hospitalized 5-7 days. More and more are being used the aortic endoprostheses, after the first intervention performed by Parodi in 1991, with the aim of achieving a reduction of the mortality and morbidity, and a less serious post-operative course.
First of all, the endoprosthesis is recommended for patients with surgical access problems (hostile abdomen) or at high surgical risk, or in the elderly with a favourable aneurysm anatomical configuration. This last issue mostly concerns the aortic neck, in relation to its anatomical and wall features and its susceptibility to the enlargement.

The new generation of endoprostheses allow absolutely better results thank to the enhanced bio-engineering plan that makes it possible to rely suprarenal free stent, modular structure, the availability of extender cuffs and the miniaturization of releasing devices. The endoprostheses are introduced by inguinal way through the femoral artery without opening the abdomen, and in the most of the patients the intervention may be performed under epidural anesthesia.
The surgery mortality for endovascular interventions is estimated around 1%, the post-operative course is less painful and demanding, and the patient may be discharged after 1-2 days.
As therapeutic indication, the endovascular surgery is really beneficial, especially in patients at high risk. In these subjects - identified for the age > 70 years, the presence of at least one co-morbidity factor (symptomatic heart failure, heart valvular disease, cardiac arrhythmia, respiratory distress or chronic renal failure) and an aneurysm diameter of at least 5.5 cm - the endovascular restoration allows to lower the perioperative mortality from 5.1% on open surgery to 2.9%, according to the data recently shown in the EVAR2 study.
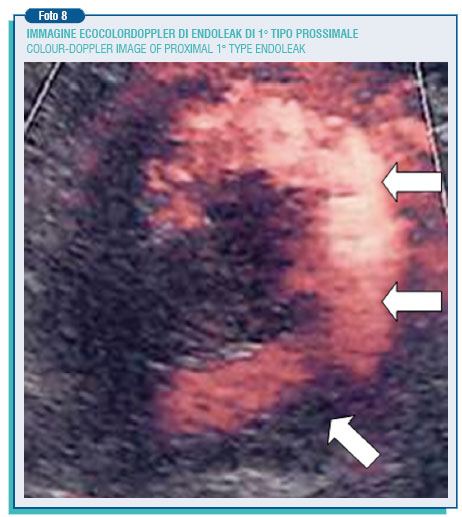
However, during the follow-up period the aneurysm may not reduce its dimension or even increase it and this as result of problems relate to endoleaks and endotension. It is named endoleak the presence of blood flow in the space between the endoprosthesis and the aortic wall.
This persisting flow leads to the aneurysm enlargement which increase the risk of the aorta break-up in spite of the surgery.
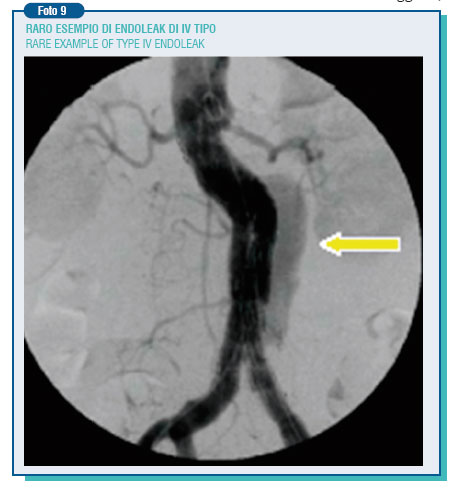
The endoleak currently represents the weak point of abdominal endovascular techniques. In the published data, its incidence is extremely varying (0-44%). The endoleak can be the result of an incomplete adhesion of the endoprosthesis to the aortic wall (1st type endoleak), originate from a lumbar artery or from the inferior mesenteric artery, going to supply in a retrograde way the aneurysm, cut off by the prosthesis - (2 nd type endoleak), or be due to prosthesis damages or porosity (3rd and 4th type endoleak).
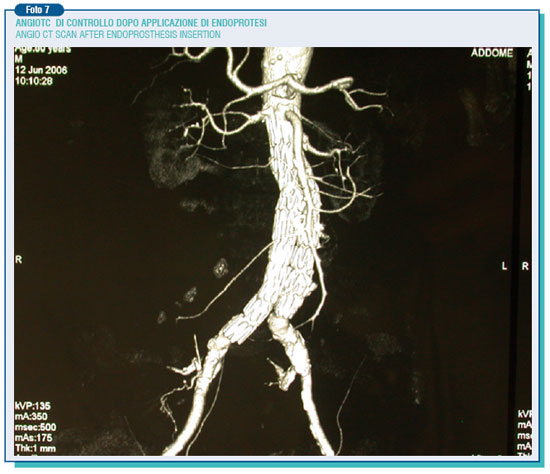
The endoleaks may also occur afterwards (secondary endoleaks), likely as a consequence of the breakdown of the aortic wall on which lies the radial burden of the prosthesis (mainly its metallic structure), or because of the prosthesis movement. On the other hand the endotension represents a steady or recurring pressure in the aneurysmatic pouch after the endoprosthesis surgery. For this reason, it can be classified on the basis of the residual pouch flow and the ensuing pressure. Therefore, an high pressure high flow endotension arises from type 1th endoleaks, high pressure low flow endotension from type 2nd endoleaks. However, even a tricky endotension at low pressure and flow, by mechanisms still to be elucidate, may happen due to the persistence of a pressure on a seemingly well sealed pouch, transmitted through the sphygmic wave. Therefore, these phenomena require a thorough and scheduled follow-up in order to monitor possible aneurysm modifications during the after surgery period. Indeed, while for the traditional intervention follow-up is better, as a rule, to perform on ultrasound of the abdominal aorta once a year for about 4-5 years, when it comes to the endoprosthesis intervention is needed to perform an angio CT scan every year for the whole life span. Anyway, the evidence of the growth of the residual aneurysm must put the operators on alert pushing the patient to undergo comprehensive exams to identify and treat possible causes.
A third approach to the therapy of AAAs is the mini-invasive aortic surgery that may consist of a mini-laparotomy or video-laparoscopy. Generally, the mini-laparotomy address the aneurysms by the retroperitoneal access through a left transversal incision of around 5 cm below the ribs or a median longitudinal incision of the same length.
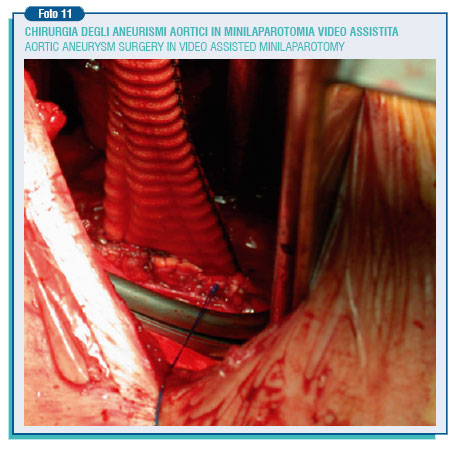
This kind of surgery means reducing the dangerousness and the post-operative hospitalization, with good medium-term results. Less used is the trans-peritoneal access with a supraumbilical longitudinal or transversal incision of 10-12 cm., or the left lumbar sideways incision of 8 -12 cm. However, to perform these interventions it is essential to have a particular surgical set and, most important, the surgeon be accomplished with operating in a very confined field. This is just the very contrary of what had been stated once: great incision, great surgeon.
A still greater training is required for the aortic video-laparoscopic procedure which is carried out through six small openings in the abdomen, through which are introduced the video camera and tool arrays like scissors and needle-holders, forceps, aspirator, proximal and distal clamps. Even though the first intervention was performed by Dion in 1993 such a technique is still remaining underused, as surgical time procedures are longer and it can be performed only in selected cases: absence of calcifications and anatomical abnormalities, non obese individuals etc. Usually, it is chosen the transperitoneal retrocolic access.
The usefulness of laparoscopy in treating AAAs is not yet clear, because the restoration is difficult not only in suturing, but also for the exposure, dissection, and clip haemostasis of the lumbar arteries. The association between videolaparoscopy and mini-laparotomy assists, speeds and makes safer the intervention (the so called hand assisted laparoscopy). In general the way of access is transperitoneal with a supraumbilical minilaparotomy of 6-8 cm., and the introduction into the abdomen of the surgeon's non-dominant hand. Also the retroperitoneal access is possible, with a minilaparotomy of 4 cm made between the anterior-superior iliac wing and the 12th rib or below the ribs on the left side. This third approach along with the endovascular meets the need of the vascular surgery evolution in a mini-invasive sense, waiting for possible future contributions coming from the robotics surgery.
However, they are not expected to be a substitute to the traditional surgery, whose results are still remaining the gold standard.
I wish ending to underline again the need of operating, according with what Crawford thought in 1982: the first risk factor of an aneurysm is the presence of the aneurysm. Regularly checking its evolution simply means to observe its expansion; over 5 cm. it means to wait for its break-up.
Prof. Giorgio Agrifoglio
Chair of Vascular Surgery
Università degli studi di Milano
Bibliografia
1. Agrifoglio G., Gabrielli L., Chirurgia degli aneurismi dell'aorta e dei suoi rami. CIC, Roma, 1989
2. Alimi YS, Hartung O, Valerio N, Juhan C. Laparoscopic aortoiliac surgery for aneurysm and occlusive disease: when should a minilaparotomy be performed?. J Vasc Surg. 33: 469, 2001.
3. Anderson PL, Arons RR, Moskowitz AJ, Gelijns A, Magnell C, Faries PL, Clair D, Nowygrod R, Kent KC. A statewide experience with endovascular abdominal aortic aneurysm repair: rapid diffusion with excellent early results. J Vasc Surg. 39: 10, 2004.
4. Bergan J.J., Yao J.S.T.: Aneurysms: diagnosis and treatment. Grune & Stratton, New York, 1982.
5. Carpenter JP, for the Endologix Investigators. Multicenter trial of the PowerLink bifurcated system for endovascular aortic aneurysm repair. J. Vasc. Surg. 36: 1129, 2002.
6. Castronuovo JJ Jr, James KV, Resnikoff M, McLean ER, Edoga JK. Laparoscopic-assisted abdominal aortic aneurysmectomy. J Vasc Surg. 32: 224, 2000.
7. Creech O. Jr:
Endoaneurysmorraphy and treatment of aortic aneurysms. Ann Surg. 164: 935, 1966.
8. Dion YM, Gracia CR, Ben El Kadi H H. Totally laparoscopic abdominal aortic aneurysm repair. J Vasc Surg. 33: 181, 2001.
9. Dubost C, Allary M, Oeconomos N.: Resection of an aneurysm of the abdominal aorta: reestablishment of the continuity by a preserved human arterial graft, with result after five months. Arch Surg. 64:405, 1952.
10. Lederle FA, Johnson GR, Wilson SE et Al. for the Veterans Affairs Cooperative Study #417 Investigators Rupture Rate of Large Abdominal Aortic Aneurysms in Patients Refusing or Unfit for Elective Repair. JAMA. 287: 2968, 2002.
11. Lifeline Registry of EVAR Publications Committee Lifeline registry of endovascular aneurysm repair: long-term primary outcome measures. J Vasc Surg. 42: 1, 2005.
12. Matsumura JS, Brewster DC, Makaroun MS, Naftel DC. A multicenter controlled clinical trial of open versus endovascular treatment of abdominal aortic aneurysm. J Vasc Surg. 37: 262, 2003.
13. Moore WS, Matsumura JS, Makaroun MS, et al; EVT/Guidant Investigators: Five-year interim comparison of the Guidant bifurcated endograft with open repair of abdominal aortic aneurysm. J Vasc Surg 38: 46, 2003
14. Parodi J.C., Barone A.P., Schonholz C. Endovascular Treatment of Abdominal Aortic Aneurysms: Lessons Learned. J. Endovasc. Surg. 4: 102, 1997.
15. Sicard GA, Zwolak RM, Sidawy AN, White RA, Siami FS: Endovascular abdominal aortic aneurysm repair: Long-term outcome measures in patients at high-risk for open surgery. J.Vasc. Surg. 44: 229, 2006.



